Neuromodulatory Selection of Motor Neuron Recruitment Patterns in a Visuomotor Behavior Increases Speed
- PMID: 32084402
- PMCID: PMC7611532
- DOI: 10.1016/j.cub.2019.12.064
Neuromodulatory Selection of Motor Neuron Recruitment Patterns in a Visuomotor Behavior Increases Speed
Abstract
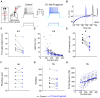
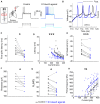
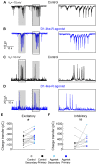
Animals generate locomotion at different speeds to suit their behavioral needs. Spinal circuits generate locomotion at these varying speeds by sequential activation of different spinal interneurons and motor neurons. Larval zebrafish can generate slow swims for prey capture and exploration by activation of secondary motor neurons and much faster and vigorous swims during escape and struggle via additional activation of primary motor neurons. Neuromodulators are known to alter the motor output of spinal circuits, but their precise role in speed regulation is not well understood. Here, in the context of optomotor response (OMR), an innate evoked locomotor behavior, we show that dopamine (DA) provides an additional layer to regulation of swim speed in larval zebrafish. Activation of D1-like receptors increases swim speed during OMR in free-swimming larvae. By analyzing tail bend kinematics in head-restrained larvae, we show that the increase in speed is actuated by larger tail bends. Whole-cell patch-clamp recordings from motor neurons reveal that, during OMR, typically only secondary motor neurons are active, whereas primary motor neurons are quiescent. Activation of D1-like receptors increases intrinsic excitability and excitatory synaptic drive in primary and secondary motor neurons. These actions result in greater recruitment of motor neurons during OMR. Our findings provide an example of neuromodulatory reconfiguration of spinal motor neuron speed modules where members are selectively recruited and motor drive is increased to effect changes in locomotor speed. VIDEO ABSTRACT.
Keywords: D1-like receptor; central pattern generator; dopamine; excitability; locomotion; optomotor response; spinal cord; swimming; synaptic; zebrafish.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Comment in
-
Motor Control: Swim Harder, Faster, Stronger.Curr Biol. 2020 Mar 9;30(5):R229-R232. doi: 10.1016/j.cub.2020.01.008. Curr Biol. 2020. PMID: 32155428
References
-
- McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71. - PubMed
-
- Gosgnach S, Lanuza GM, Butt SJB, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

