Evaluation of systemic inflammatory response and lung injury induced by Crotalus durissus cascavella venom
- PMID: 32084665
- PMCID: PMC7035002
- DOI: 10.1371/journal.pone.0224584
Evaluation of systemic inflammatory response and lung injury induced by Crotalus durissus cascavella venom
Abstract
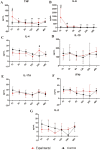
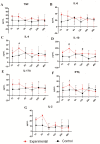
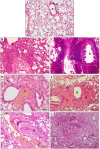
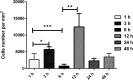
This study investigated the systemic inflammatory response and mechanism of pulmonary lesions induced by Crotalus durissus cascavella venom in murine in the state of Bahia. In order to investigate T helper Th1, Th2 and Th17 lymphocyte profiles, we measured interleukin (IL) -2, IL-4, IL-6, IL-10, IL-17, tumor necrosis factor (TNF) and interferon gamma (IFN-γ) levels in the peritoneal fluid and macerated lungs of mice and histopathological alterations at the specific time windows of 1h, 3h, 6h, 12h, 24h and 48h after inoculation with Crotalus durissus cascavella venom. The data demonstrated an increase of acute-phase cytokines (IL-6 and TNF) in the first hours after inoculation, with a subsequent increase in IL-10 and IL-4, suggesting immune response modulation for the Th2 profile. The histopathological analysis showed significant morphological alterations, compatible with acute pulmonary lesions, with polymorphonuclear leukocyte (PMN) infiltration, intra-alveolar edema, congestion, hemorrhage and atelectasis. These findings advance our understanding of the dynamics of envenomation and contribute to improve clinical management and antiophidic therapy for individuals exposed to venom.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. Rabies and envenomings. A neglected public health issue: report of a consultative meeting. [Internet]. World Health Organization, editor. Geneva; 2007. 32 p. Available from: https://apps.who.int/iris/handle/10665/43858
-
- Barravieira B, Lomonte B, Tarkowski A, Hanson LÃ, Meira DA. Acute-phase reactions, including cytokines, in patients bitten by Bothrops and Crotalus snakes in Brazil. J Venom Anim Toxins [Internet]. 1995;1:11–22. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79301995000...
-
- Biondi I. Caracterização biológica e bioquímica da peçonha de Crotalus durissus cascavella no Estado da Bahia. State University of Feira de Santana; 2009.
-
- Petricevich VL, Teixeira CFP, Tambourgi D V, Gutiérrez JM. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon [Internet]. 2000;38(9):1253–66. Available from: http://www.sciencedirect.com/science/article/pii/S0041010199002275 10.1016/s0041-0101(99)00227-5 - DOI - PubMed
-
- Luna K, Melo C, Pascoal VPM, Martins-Filho OA, Pereira VRA. Bothrops erythromelas snake venom induces a proinflammatory response in mice splenocytes. Int J Interf Cytokine Mediat Res. 2011. January 1;3:9–18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

