Supramolecular Sandwiches: Halogen-Bonded Coformers Direct [2+2] Photoreactivity in Two-Component Cocrystals
- PMID: 32085511
- PMCID: PMC7070622
- DOI: 10.3390/molecules25040907
Supramolecular Sandwiches: Halogen-Bonded Coformers Direct [2+2] Photoreactivity in Two-Component Cocrystals
Abstract
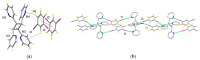
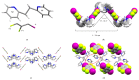
The halogen-bond (X-bond) donors 1,3- and 1,4-diiodotetrafluorobenzene (1,3-di-I-tFb and 1,4-di-I-tFb, respectively) form cocrystals with trans-1,2-bis(2-pyridyl)ethylene (2,2'-bpe) assembled by N···I X-bonds. In each cocrystal, 2(1,3-di-I-tFb)·2(2,2'-bpe) and (1,4-di-I-tFb)·(2,2'-bpe), the donor molecules support the C=C bonds of 2,2'-bpe to undergo an intermolecular [2+2] photodimerization. UV irradiation of each cocrystal resulted in stereospecific and quantitative conversion of 2,2'-bpe to rctt-tetrakis(2-pyridyl)cyclobutane (2,2'-tpcb). In each case, the reactivity occurs via face-to-face π-stacked columns wherein nearest-neighbor pairs of 2,2'-bpe molecules lie sandwiched between X-bond donor molecules. Nearest-neighbor C=C bonds are stacked criss-crossed in both cocrystals. The reactivity was ascribed to the olefins undergoing pedal-like motion in the solid state. The stereochemistry of 2,2'-tpcb is confirmed in cocrystals 2(1,3-di-I-tFb)·(2,2'-tpcb) and (1,4-di-I-tFb)·(2,2'-tpcb).
Keywords: cocrystal; crystal engineering; cyclobutane; halogen bonding; pedal motion.; photodimerization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

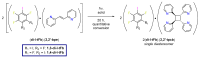


Similar articles
-
Halogen-Bond Mediated [2+2] Photodimerizations: À la Carte Access to Unsymmetrical Cyclobutanes in the Solid State.Molecules. 2022 Feb 3;27(3):1048. doi: 10.3390/molecules27031048. Molecules. 2022. PMID: 35164313 Free PMC article.
-
Halogen versus Hydrogen Bonding in Binary Cocrystals: Novel Conformation a Coformer with [2+2] Photoreactivity of Criss-Crossed C=C Bonds.Chemphyschem. 2020 Jan 16;21(2):154-163. doi: 10.1002/cphc.201900961. Epub 2019 Dec 9. Chemphyschem. 2020. PMID: 31600417
-
Crystal structure and photoreactivity of a halogen-bonded cocrystal based upon 1,2-diiodoperchlorobenzene and 1,2-bis(pyridin-4-yl)ethylene.Acta Crystallogr C Struct Chem. 2020 Jun 1;76(Pt 6):557-561. doi: 10.1107/S2053229620006233. Epub 2020 May 13. Acta Crystallogr C Struct Chem. 2020. PMID: 32499452
-
Rediscovery of halogen bonds in protein-ligand complexes.Mini Rev Med Chem. 2010 Apr;10(4):309-14. doi: 10.2174/138955710791331016. Mini Rev Med Chem. 2010. PMID: 20470246 Review.
-
Relationships between hydrogen bonds and halogen bonds in biological systems.Acta Crystallogr B Struct Sci Cryst Eng Mater. 2017 Apr 1;73(Pt 2):255-264. doi: 10.1107/S2052520617003109. Epub 2017 Mar 29. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2017. PMID: 28362290 Review.
Cited by
-
Halogen-Bond Mediated [2+2] Photodimerizations: À la Carte Access to Unsymmetrical Cyclobutanes in the Solid State.Molecules. 2022 Feb 3;27(3):1048. doi: 10.3390/molecules27031048. Molecules. 2022. PMID: 35164313 Free PMC article.
-
Topochemical, Single-Crystal-to-Single-Crystal [2+2] Photocycloadditions Driven by Chalcogen-Bonding Interactions.Angew Chem Int Ed Engl. 2022 Sep 5;61(36):e202206249. doi: 10.1002/anie.202206249. Epub 2022 Jul 27. Angew Chem Int Ed Engl. 2022. PMID: 35797220 Free PMC article.
-
Complementary, Cooperative Ditopic Halogen Bonding and Electron Donor-Acceptor π-π Complexation in the Formation of Cocrystals.Molecules. 2022 Feb 24;27(5):1527. doi: 10.3390/molecules27051527. Molecules. 2022. PMID: 35268629 Free PMC article.
References
-
- Allen F.H., Goud B.S., Hoy V.J., Howard J.A.K., Desiraju G.R. Molecular Recognition via Iodo·Nitro and Iodo·Cyano Interactions: Crystal Structures of the 1:1 Complexes of 1, 4-Diiodobenzene with 1, 4-Dinitrobenzene and 7, 7, 8, 8-Tetracyanoquinodimethane (TCNQ) J. Chem. Soc. Chem. Commun. 1994;23:2729–2730. doi: 10.1039/c39940002729. - DOI
-
- Reddy D.S., Craig D.C., Rae A.D., Desiraju G.R. N···Br Mediated Diamondoid Network in the Crystalline Complex Carbon Tetrabromide: Hexamethylenetetramine. J. Chem. Soc. Chem. Commun. 1993;23:1737–1739. doi: 10.1039/c39930001737. - DOI
-
- Desiraju G.R., Ho P.S., Kloo L., Legon A.C., Marquardt R., Metrangolo P., Politzer P., Resnati G., Rissanen K. Definition of the Halogen Bond (IUPAC recommendations 2013) Pure Appl. Chem. 2013;85:1711–1713. doi: 10.1351/PAC-REC-12-05-10. - DOI
-
- Marras G., Metrangolo P., Meyer F., Pilati T., Resnati G., Vij A. Solid State Synthesis Under Supramolecular Control of a 2D Heterotetratopic Self-Complementary Tecton Tailored to Halogen Bonding. New J. Chem. 2006;30:1397–1402. doi: 10.1039/b605958a. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

