The Major Cat Allergen Fel d 1 Binds Steroid and Fatty Acid Semiochemicals: A Combined In Silico and In Vitro Study
- PMID: 32085519
- PMCID: PMC7073184
- DOI: 10.3390/ijms21041365
The Major Cat Allergen Fel d 1 Binds Steroid and Fatty Acid Semiochemicals: A Combined In Silico and In Vitro Study
Abstract
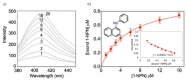
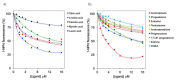
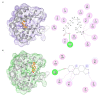
The major cat allergen Fel d 1 is a tetrameric glycoprotein of the secretoglobin superfamily. Structural aspects and allergenic properties of this protein have been investigated, but its physiological function remains unclear. Fel d 1 is assumed to bind lipids and steroids like the mouse androgen-binding protein, which is involved in chemical communication, either as a semiochemical carrier or a semiochemical itself. This study focused on the binding activity of a recombinant model of Fel d 1 (rFel d 1) towards semiochemical analogs, i.e., fatty acids and steroids, using both in silico calculations and fluorescence measurements. In silico analyses were first adopted to model the interactions of potential ligands, which were then tested in binding assays using the fluorescent reporter N-phenyl-1-naphthylamine. Good ligands were fatty acids, such as the lauric, oleic, linoleic, and myristic fatty acids, as well as steroids like androstenone, pregnenolone, and progesterone, that were predicted by in silico molecular models to bind into the central and surface cavities of rFel d 1, respectively. The lowest dissociation constants were shown by lauric acid (2.6 µM) and androstenone (2.4 µM). The specific affinity of rFel d 1 to semiochemicals supports a function of the protein in cat's chemical communication, and highlights a putative role of secretoglobins in protein semiochemistry.
Keywords: 2D interaction maps; N-phenyl-1-naphthylamine; chemical communication; in silico docking; ligand-binding assays; molecular modeling; odorant-binding protein; pheromone; protein–ligand interactions; secretoglobin.
Conflict of interest statement
Patrick Pageat is the inventor of the patent “Properties of cat’s facial pheromone” n° WO1996023414A1 and the patent “Cat Appeasing Pheromone” n° WO2015140631A1.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

