Efficacy and Safety of OnabotulinumtoxinA 400 Units in Patients with Post-Stroke Upper Limb Spasticity: Final Report of a Randomized, Double-Blind, Placebo-Controlled Trial with an Open-Label Extension Phase
- PMID: 32085529
- PMCID: PMC7077183
- DOI: 10.3390/toxins12020127
Efficacy and Safety of OnabotulinumtoxinA 400 Units in Patients with Post-Stroke Upper Limb Spasticity: Final Report of a Randomized, Double-Blind, Placebo-Controlled Trial with an Open-Label Extension Phase
Abstract
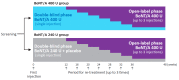
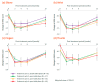
In many countries, 400 units (U) is the maximum dose of onabotulinumtoxinA available to treat upper limb spasticity, but few studies have demonstrated the optimal use of this dose. In the double-blind phase of this randomized, controlled trial, we compared the efficacy and safety of 400 vs. 240 U onabotulinumtoxinA in patients with post-stroke upper limb spasticity. Both groups received 240 U onabotulinumtoxinA injected in the forearm. An additional 160 U onabotulinumtoxinA (400 U group) or placebo (240 U group) was injected in the elbow flexors. Both groups showed similar muscle tone reduction in the wrist, fingers, and thumb; muscle tone reduction in the elbow flexors was greater in the group treated with onabotulinumtoxinA (400 U group) compared to placebo (240 U group). Functional disabilities improved in both groups. No substantial difference was found in safety profiles. In the subsequent open-label phase, all participants received repeat injections of 400 U onabotulinumtoxinA (target muscles and doses per muscle determined by the physician). Similar efficacy and safety outcomes, as with the 400 U group in the double-blind phase, were confirmed. This final report demonstrates that injection of onabotulinumtoxinA 400 U relieves muscle tone in a wide range of areas and improves functional disabilities; generally, it was well-tolerated, and no new safety concerns were identified. The dosing data in the open-label phase will inform optimal use of onabotulinumtoxinA in clinical practice (ClinicalTrials.gov: NCT03261167).
Keywords: botulinum toxin; randomized controlled trial; stroke; upper limb spasticity.
Conflict of interest statement
This study (NCT03261167) was conducted with a research fund from GlaxoSmithKline K.K., in which Masahiro Abo, Takashi Shigematsu and Hiroyoshi Hara participated as principal investigator and their institutions received funding in relation to this work. Yasuko Matsuda, Akinori Nimura, Yoshiyuki Yamashita and Kaoru Takahashi are employees of GlaxoSmithKline K.K. OnabotulinumtoxinA was provided by Allergan plc.
Figures
References
-
- Lance J.W. Symposium synopsis. In: Feldman R.G., Young R.R., Koella W.P., editors. Spasticity: Disordered Motor Control. Year Book Medical Publishers; Chicago, IL, USA: 1980. pp. 485–494.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

