Identification of a Neisseria gonorrhoeae Histone Deacetylase: Epigenetic Impact on Host Gene Expression
- PMID: 32085531
- PMCID: PMC7168274
- DOI: 10.3390/pathogens9020132
Identification of a Neisseria gonorrhoeae Histone Deacetylase: Epigenetic Impact on Host Gene Expression
Abstract
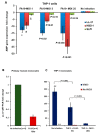
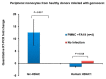
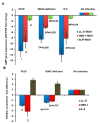
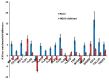
Epigenetic reprogramming in macrophages is termed trained innate immunity, which regulates immune tolerance and limits tissue damage during infection. Neisseria gonorrhoeae is a strict human pathogen that causes the sexually transmitted infection termed gonorrhea. Here, we report that this pathogen harbors a gene that encodes a histone deacetylase-like enzyme (Gc-HDAC) that shares high 3D-homology to human HDAC1, HDAC2 and HDAC8. A Gc-HDAC null mutant was constructed to determine the biologic significance of this gene. The results showed that WT gonococci reduced the expression of host defense peptides LL-37, HBD-1 and SLPI in macrophages when compared to its Gc-HDAC-deficient isogenic strain. The enrichment of epigenetic marks in histone tails control gene expression and are known to change during bacterial infections. To investigate whether gonococci exert epigenetic modifications on host chromatin, the enrichment of acetylated lysine 9 in histone 3 (H3K9ac) was investigated using the TLR-focused ChIP array system. The data showed that infection with WT gonococci led to higher H3K9ac enrichment at the promoters of pro-inflammatory mediators' genes, many TLRs, adaptor proteins and transcription factors, suggesting gene activation when compared to infection with the Gc-HDAC-deficient mutant. Taken together, the data suggest that gonococci can exert epigenetic modifications on host cells to modulate certain macrophage defense genes, leading to a maladaptive state of trained immunity.
Keywords: H3K9ac; HDAC; Neisseria gonorrhoeae; chemokines; cytokines; epigenetic; gonorrhea; infection; macrophage; survival.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Rowley J., Vander Hoorn S., Korenromp E., Low N., Unemo M., Abu-Raddad L.J., Chico R.M., Smolak A., Newman L., Gottlieb S., et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019;97:548–562. doi: 10.2471/BLT.18.228486. - DOI - PMC - PubMed
-
- CDC Trends in aging—United States and worldwide. MMWR Morb. Mortal. Wkly. Rep. 2003;52:101–104. - PubMed
-
- Tapsall J.W., Shultz T., Limnios E., Munro R., Mercer J., Porritt R., Griffith J., Hogg G., Lum G., Lawrence A., et al. Surveillance of antibiotic resistance in invasive isolates of Neisseria meningitidis in Australia 1994–1999. Pathology. 2001;33:359–361. doi: 10.1080/pat.33.3.359.361. - DOI - PubMed
-
- Shultz T.R., Tapsall J.W., White P.A. Correlation of in vitro susceptibilities to newer quinolones of naturally occurring quinolone-resistant Neisseria gonorrhoeae strains with changes in GyrA and ParC. Antimicrob. Agents Chemother. 2001;45:734–738. doi: 10.1128/AAC.45.3.734-738.2001. - DOI - PMC - PubMed
-
- WHO . Emergence of Multi-Drug Resistant Neisseria Gonorrhoeae—Threat of Global Rise in Untreated Sexually Transmitted Infections. WHO; Geneva, Switzerland: 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

