The Role of Gamma Delta T Cells in Autoimmune Rheumatic Diseases
- PMID: 32085540
- PMCID: PMC7072729
- DOI: 10.3390/cells9020462
The Role of Gamma Delta T Cells in Autoimmune Rheumatic Diseases
Abstract
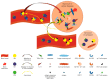
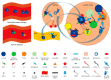
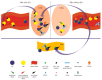
Autoimmune rheumatic diseases (ARDs), affecting ~1-1.5% of all humans, are associated with considerable life long morbidity and early mortality. Early studies in the 1990s showed numerical changes of the recently discovered γδ T cells in the peripheral blood and in affected tissues of patients with a variety of ARDs, kindling interest in their role in the immuno-pathogenesis of these chronic inflammatory conditions. Indeed, later studies applied rapid developments in the understanding of γδ T cell biology, including antigens recognized by γδ T cells, their developmental programs, states of activation, and cytokine production profiles, to analyze their contribution to the pathological immune response in these disorders. Here we review the published studies addressing the role of γδ T in the major autoimmune rheumatic diseases, including rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, systemic lupus erythematosus and scleroderma, and animal models thereof. Due to their unique properties spanning adaptive and innate immune functions, the ever deeper understanding of this unique T cell population is shedding new light on the pathogenesis of, while potentially enabling new therapeutic approaches to, these diseases.
Keywords: ankylosing spondylitis; gammadelta T cells; juvenile idiopathic arthritis; rheumatoid arthritis; systemic lupus erythematosus; systemic sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Lymphocyte transformation to connective tissue antigens in adult and juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, systemic lupus erythematosus, and a nonarthritic control population.Cell Immunol. 1983 Nov;82(1):196-209. doi: 10.1016/0008-8749(83)90153-3. Cell Immunol. 1983. PMID: 6640674
-
Review: Innate Lymphoid Cells: Sparking Inflammatory Rheumatic Disease?Arthritis Rheumatol. 2017 May;69(5):885-897. doi: 10.1002/art.40068. Arthritis Rheumatol. 2017. PMID: 28217945 Review. No abstract available.
-
The clinical significance of titered antinuclear antibodies.Arthritis Rheum. 1967 Dec;10(6):544-52. doi: 10.1002/art.1780100607. Arthritis Rheum. 1967. PMID: 4864485 No abstract available.
-
Expression of the CD2 activation epitope T11-3 (CD2R) on T cells in rheumatoid arthritis, juvenile rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, and Lyme disease: phenotypic and functional analysis.Scand J Immunol. 1991 Sep;34(3):351-8. doi: 10.1111/j.1365-3083.1991.tb01556.x. Scand J Immunol. 1991. PMID: 1715605
-
The immunology of rheumatoid diseases.Clin Symp. 1979;31(4):1-36. Clin Symp. 1979. PMID: 45382 Review. No abstract available.
Cited by
-
CD19 CAR-T cell therapy: a new dawn for autoimmune rheumatic diseases?Front Immunol. 2024 Dec 17;15:1502712. doi: 10.3389/fimmu.2024.1502712. eCollection 2024. Front Immunol. 2024. PMID: 39742256 Free PMC article. Review.
-
Identification biomarkers and therapeutic targets of disulfidptosis-related in rheumatoid arthritis via bioinformatics, molecular dynamics simulation, and experimental validation.Sci Rep. 2025 Mar 13;15(1):8779. doi: 10.1038/s41598-025-93656-4. Sci Rep. 2025. PMID: 40082645 Free PMC article.
-
Clonal associations between lymphocyte subsets and functional states in rheumatoid arthritis synovium.Nat Commun. 2024 Jun 11;15(1):4991. doi: 10.1038/s41467-024-49186-0. Nat Commun. 2024. PMID: 38862501 Free PMC article.
-
Mechanisms of γδ T cell accumulation in visceral adipose tissue with aging.Front Aging. 2024 Jan 11;4:1258836. doi: 10.3389/fragi.2023.1258836. eCollection 2023. Front Aging. 2024. PMID: 38274288 Free PMC article.
-
Blood transcriptomics to facilitate diagnosis and stratification in pediatric rheumatic diseases - a proof of concept study.Pediatr Rheumatol Online J. 2022 Oct 17;20(1):91. doi: 10.1186/s12969-022-00747-x. Pediatr Rheumatol Online J. 2022. PMID: 36253751 Free PMC article.
References
-
- Acuto O., Hussey R.E., Fitzgerald K.A., Protentis J.P., Meuer S.C., Schlossman S.F., Reinherz E.L. The human T cell receptor: Appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983;34:717–726. doi: 10.1016/0092-8674(83)90528-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

