Selected Fungal Natural Products with Antimicrobial Properties
- PMID: 32085562
- PMCID: PMC7070998
- DOI: 10.3390/molecules25040911
Selected Fungal Natural Products with Antimicrobial Properties
Abstract
Fungal natural products and their effects have been known to humankind for hundreds of years. For example, toxic ergot alkaloids produced by filamentous fungi growing on rye poisoned thousands of people and livestock throughout the Middle Ages. However, their later medicinal applications, followed by the discovery of the first class of antibiotics, penicillins and other drugs of fungal origin, such as peptidic natural products, terpenoids or polyketides, have altered the historically negative reputation of fungal "toxins". The development of new antimicrobial drugs is currently a major global challenge, mainly due to antimicrobial resistance phenomena. Therefore, the structures, biosynthesis and antimicrobial activity of selected fungal natural products are described here.
Keywords: antimicrobial properties; antimicrobial resistance; biosynthesis; ergot alkaloids; fungal metabolites; natural products; peptides; polyketides; terpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
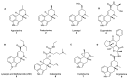
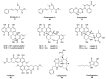
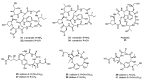


References
-
- Dewick P.M. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons; West Sussex, UK: 2002.
-
- Mukherjee J., Menge M. New Products and New Areas of Bioprocess Engineering. Springer; Berlin/Heidelberg, Germany: 2000. Progress and prospects of ergot alkaloid research; pp. 1–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

