Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues
- PMID: 32085577
- PMCID: PMC7075292
- DOI: 10.3390/nano10020355
Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues
Abstract
(1) Background: Carprofen (CP), 2-(6-chlorocarbazole) propionic acid, is used as an anti-inflammatory, analgesic and anti-pyretic agent and it belongs to the family of non-steroidal anti-inflammatory drugs (NSAIDs). CP has some adverse reactions in systemic administration; for this reason, topical administration with CP nanoparticles (CP-NPs) can be an optimal alternative. The main objective of this work is the investigation of ex vivo permeation of CP through different types of porcine mucous membranes (buccal, sublingual and vaginal) and ophthalmic tissues (cornea, sclera and conjunctiva) to compare the influence of CP-NPs formulation over a CP solution (CP-Solution). (2) Methods: The ex vivo permeation profiles were evaluated using Franz diffusion cells. Furthermore, in vivo studies were performed to verify that the formulations did not affect the cell structure and to establish the amount retained (Qr) in the tissues. (3) Results: Permeation of CP-NPs is more effective in terms of drug retention in almost all tissues (with the exception of sclera and sublingual). In vivo studies show that neither of the two formulations affects tissue structure, so both formulations are safe. (4) Conclusions: It was concluded that CP-NPs may be a useful tool for the topical treatment of local inflammation in veterinary and human medicine.
Keywords: NSAIDs; anti-inflammatory; carprofen; drug delivery system; nanoparticles; solution; veterinary diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
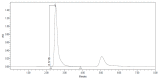
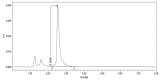
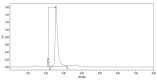
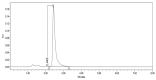
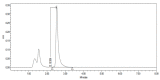
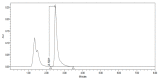
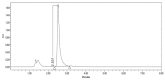






References
-
- Committe for Veterinary Medicinal Products Carpronfen Summary Report. The European Agency for the Evaluation of Medicinal Products; London, UK: 1999. EMEA/MRL/042/95-FINAL.
LinkOut - more resources
Full Text Sources
Miscellaneous

