Fluorescent Light Energy (FLE) Acts on Mitochondrial Physiology Improving Wound Healing
- PMID: 32085605
- PMCID: PMC7073965
- DOI: 10.3390/jcm9020559
Fluorescent Light Energy (FLE) Acts on Mitochondrial Physiology Improving Wound Healing
Abstract
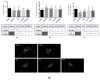
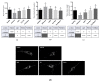
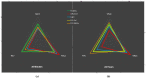
Fluorescent light energy (FLE) has been used to treat various injured tissues in a non-pharmacological and non-thermal fashion. It was applied to stimulate cell proliferation, accelerate healing in chronic and acute wounds, and reduce pain and inflammation. FLE has been shown to reduce pro-inflammatory cytokines while promoting an environment conducive to healing. A possible mechanism of action of FLE is linked to regulation of mitochondrial homeostasis. This work aims to investigate the effect of FLE on mitochondrial homeostasis in an in vitro model of inflammation. Confocal microscopy and gene expression profiling were performed on cultures of inflamed human dermal fibroblasts treated with either direct light from a multi-LED lamp, or FLE from either an amorphous gel or sheet hydrogel matrix. Assessment using confocal microscopy revealed mitochondrial fragmentation in inflamed cells, likely due to exposure to inflammatory cytokines, however, mitochondrial networks were restored to normal 24-h after treatment with FLE. Moreover, gene expression analysis found that treatment with FLE resulted in upregulation of uncoupling protein 1 (UCP1) and carnitine palmitoyltransferase 1B (CPT1B) genes, which encode proteins favoring mitochondrial ATP production through oxidative phosphorylation and lipid β-oxidation, respectively. These observations demonstrate a beneficial effect of FLE on mitochondrial homeostasis in inflamed cells.
Keywords: fluorescence; fluorescent light energy; gene expression; inflammation; mitochondria; mitochondrial dynamics; wound healing.
Conflict of interest statement
Drs. Zago, Campbell, Hébert, and Nielsen are employees of Klox Technologies. The other authors have no conflict of interest relevant to the content of this article.
Figures
Similar articles
-
Evaluation of Fluorescent Light Energy for the Treatment of Acute Second-degree Burns.Mil Med. 2021 Jan 25;186(Suppl 1):416-423. doi: 10.1093/milmed/usaa299. Mil Med. 2021. PMID: 33499452
-
FLUORESCENT LIGHT ENERGY: The Future for Treating Inflammatory Skin Conditions?J Clin Aesthet Dermatol. 2019 May;12(5):E61-E68. Epub 2019 May 1. J Clin Aesthet Dermatol. 2019. PMID: 31320979 Free PMC article.
-
Fluorescent light energy modulates healing in skin grafted mouse model.Open Med (Wars). 2021 Aug 27;16(1):1240-1255. doi: 10.1515/med-2021-0329. eCollection 2021. Open Med (Wars). 2021. PMID: 34522783 Free PMC article.
-
Mitochondrial Uncoupling and the Regulation of Glucose Homeostasis.Curr Diabetes Rev. 2017;13(4):386-394. doi: 10.2174/1573399812666160217122707. Curr Diabetes Rev. 2017. PMID: 26900134 Review.
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
Cited by
-
Fluorescent light energy combined with systemic isotretinoin: A 52-week follow-up evaluating efficacy and safety in treatment of moderate-severe acne.Clin Case Rep. 2021 Feb 20;9(4):2057-2068. doi: 10.1002/ccr3.3944. eCollection 2021 Apr. Clin Case Rep. 2021. PMID: 33936640 Free PMC article.
-
Fluorescent Light Energy and Chronic Lesions: A Winning Association.Plast Reconstr Surg Glob Open. 2021 Jul 13;9(7):e3667. doi: 10.1097/GOX.0000000000003667. eCollection 2021 Jul. Plast Reconstr Surg Glob Open. 2021. PMID: 34277317 Free PMC article.
-
Biphasic dose response in the anti-inflammation experiment of PBM.Lasers Med Sci. 2023 Feb 7;38(1):66. doi: 10.1007/s10103-022-03664-3. Lasers Med Sci. 2023. PMID: 36749428 Review.
-
New perspectives in regenerative medicine and surgery: the bioactive composite therapies (BACTs).Eur J Plast Surg. 2022;45(1):1-25. doi: 10.1007/s00238-021-01874-6. Epub 2021 Oct 29. Eur J Plast Surg. 2022. PMID: 34728900 Free PMC article.
-
Coenzyme Q10 in Mitochondrial and Lysosomal Disorders.J Clin Med. 2021 May 4;10(9):1970. doi: 10.3390/jcm10091970. J Clin Med. 2021. PMID: 34064329 Free PMC article.
References
-
- Yager D.R., Chen S.M., Ward S.I., Olutoye O.O., Diegelmann R.F., Cohen I.K. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Rep. Regen. 1997;5:23–32. doi: 10.1046/j.1524-475X.1997.50108.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

