Inhibition of Angiotensin-Converting Enzyme Ameliorates Renal Fibrosis by Mitigating DPP-4 Level and Restoring Antifibrotic MicroRNAs
- PMID: 32085655
- PMCID: PMC7074526
- DOI: 10.3390/genes11020211
Inhibition of Angiotensin-Converting Enzyme Ameliorates Renal Fibrosis by Mitigating DPP-4 Level and Restoring Antifibrotic MicroRNAs
Abstract
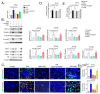
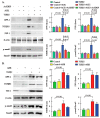
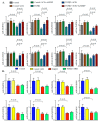
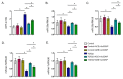
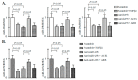
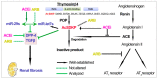
Two class of drugs 1) angiotensin-converting enzyme inhibitors (ACEis) and 2) angiotensin II receptor blockers (ARBs) are well-known conventional drugs that can retard the progression of chronic nephropathies to end-stage renal disease. However, there is a lack of comparative studies on the effects of ACEi versus ARB on renal fibrosis. Here, we observed that ACEi ameliorated renal fibrosis by mitigating DPP-4 and TGFβ signaling, whereas, ARB did not show. Moreover, the combination of N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), one of the substrates of ACE, with ACEi slightly enhanced the inhibitory effects of ACEi on DPP-4 and associated-TGFβ signaling. Further, the comprehensive miRome analysis in kidneys of ACEi+AcSDKP (combination) treatment revealed the emergence of miR-29s and miR-let-7s as key antifibrotic players. Treatment of cultured cells with ACEi alone or in combination with AcSDKP prevented the downregulated expression of miR-29s and miR-let-7s induced by TGFβ stimulation. Interestingly, ACEi also restored miR-29 and miR-let-7 family cross-talk in endothelial cells, an effect that is shared by AcSDKP suggesting that AcSDKP may be partially involved in the anti-mesenchymal action of ACEi. The results of the present study promise to advance our understanding of how ACEi regulates antifibrotic microRNAs crosstalk and DPP-4 associated-fibrogenic processes which is a critical event in the development of diabetic kidney disease.
Keywords: ACE; ARBs; AcSDKP; DPP-4; EMT; EndMT; diabetic nephropathy; kidney fibrosis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Held P.J., Port F.K., Webb R.L., Wolfe R.A., Garcia J.R., Blagg C.R., Agodoa L.Y. The united states renal data system’s 1991 annual data report: An introduction. Am. J. Kidney Dis. 1991;18:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

