Japanese encephalitis vaccination in the Philippines: A cost-effectiveness analysis comparing alternative delivery strategies
- PMID: 32085954
- PMCID: PMC7068699
- DOI: 10.1016/j.vaccine.2020.02.018
Japanese encephalitis vaccination in the Philippines: A cost-effectiveness analysis comparing alternative delivery strategies
Abstract
Introduction: Japanese encephalitis (JE) is a mosquito-borne viral infection of the brain that can cause permanent brain damage and death. In the Philippines, efforts are underway to deliver a live attenuated JE vaccine (CD-JEV) to children under five years of age (YOA), who are disproportionately infected. Multiple vaccination strategies are being considered.
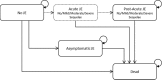
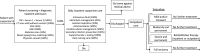
Methods: We conducted a cost-effectiveness analysis comparing three vaccination strategies to the current state of no vaccination from the societal and government perspectives: (1) national routine vaccination only, (2) sub-national campaign followed by national routine, and (3) national campaign followed by national routine. We developed a Markov model to estimate impact of vaccination or no vaccination over the child's lifetime horizon, assuming an annual incidence of 10.6 cases per 100,000. Costs of illness ($859/case), vaccine ($0.50/dose), routine vaccination ($0.95/dose), and campaign vaccination ($0.98/dose) were based on hospital financial records, expert opinion and literature. The societal perspective included transportation and opportunity costs of caregiver time, in addition to costs incurred by the health system.
Results: JE vaccination via national campaign followed by national routine delivery was the most cost-effective strategy modeled with a cost per disability adjusted life year (DALY) averted of $233 and $29 from the government and societal perspectives, respectively. Results were similar for other delivery strategies with cost/DALY ranging from $233 to $265 from the government perspective and $29-$57 from the societal perspective. JE vaccination was projected to prevent 27,856-37,277 cases, 5571-7455 deaths, and 173,233-230,704 DALYs among children under five over 20 consecutive birth cohorts. Total incremental costs of vaccination versus no vaccination over 20 birth cohorts were $6.6-$9.8 million from the societal perspective ($230 K-$440 K annually) and $45.9-$53.9 million ($2.2 M-$2.7 M annually) from the governmental perspective.
Conclusion: Vaccination with CD-JEV in the Philippines is projected to be cost-effective, reducing long-term costs associated with JE illness and improving health outcomes compared to no vaccination.
Keywords: Cost analysis; Cost-effectiveness; Japanese encephalitis; Japanese encephalitis vaccine; Philippines.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Japanese Encephalitis Vaccines WHO position paper, February 2015 - Recommendations. Vaccine. 2016;34:302–303. - PubMed
-
- PATH. Safety of CD-JEV: A proven tool for JE prevention; 2018. https://path.azureedge.net/media/documents/CVIA_Safety_CD_JEV_fs.pdf [accessed January 20, 2019].
-
- WHO Information sheet observed rate of vaccine reactions - Japanese encephalitis vaccine. Inf Sheet. 2016
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

