Extraretinal Spike Normalization in Retinal Ganglion Cell Axons
- PMID: 32086286
- PMCID: PMC7110362
- DOI: 10.1523/ENEURO.0504-19.2020
Extraretinal Spike Normalization in Retinal Ganglion Cell Axons
Abstract
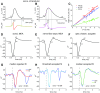
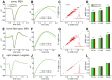
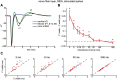
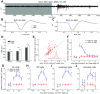
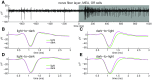
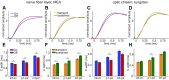
Spike conduction velocity characteristically differs between myelinated and unmyelinated axons. Here we test whether spikes of myelinated and unmyelinated paths differ in other respects by measuring rat retinal ganglion cell (RGC) spike duration in the intraretinal, unmyelinated nerve fiber layer and the extraretinal, myelinated optic nerve and optic chiasm. We find that rapid spike firing and illumination broaden spikes in intraretinal axons but not in extraretinal axons. RGC axons thus initiate spikes intraretinally and normalize spike duration extraretinally. Additionally, we analyze spikes that were recorded in a previous study of rhesus macaque retinogeniculate transmission and find that rapid spike firing does not broaden spikes in optic tract. The spike normalization we find reduces the number of spike properties that can change during RGC light responses. However, this is not because identical spikes fire in all axons. Instead, our recordings show that different subtypes of RGC generate axonal spikes of different durations and that the differences resemble spike duration increases that alter neurotransmitter release from other neurons. Moreover, previous studies have shown that RGC spikes of shorter duration can fire at higher maximum frequencies. These properties should facilitate signal transfer by different mechanisms at RGC synapses onto subcortical target neurons.
Keywords: axonal spikes; cell type-specific differences; normalization of duration; optic chiasm; optic tract; retina.
Copyright © 2020 Fogli Iseppe et al.
Figures



References
-
- Berson DM (2008) Retinal ganglion cell types and their central projections In: The senses: A comprehensive reference (Masland RH, Albright TD, eds), pp 491–520. San Diego: Academic.
