Evaluation of Quantitative Relationship Between Target Expression and Antibody-Drug Conjugate Exposure Inside Cancer Cells
- PMID: 32086295
- PMCID: PMC7153564
- DOI: 10.1124/dmd.119.089276
Evaluation of Quantitative Relationship Between Target Expression and Antibody-Drug Conjugate Exposure Inside Cancer Cells
Abstract
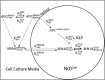
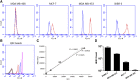
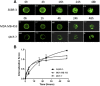
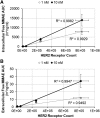
Antibody-drug conjugates (ADCs) employ overexpressed cell surface antigens to deliver cytotoxic payloads inside cancer cells. However, the relationship between target expression and ADC efficacy remains ambiguous. In this manuscript, we have addressed a part of this ambiguity by quantitatively investigating the effect of antigen expression levels on ADC exposure within cancer cells. Trastuzumab-valine-citrulline-monomethyl auristatin E was used as a model ADC, and four different cell lines with diverse levels of human epidermal growth factor receptor 2 (HER2) expression were used as model cells. The pharmacokinetics (PK) of total trastuzumab, released monomethyl auristatin E (MMAE), and total MMAE were measured inside the cells and in the cell culture media following incubation with two different concentrations of ADC. In addition, target expression levels, target internalization rate, and cathepsin B and MDR1 protein concentrations were determined for each cell line. All the PK data were mathematically characterized using a cell-level systems PK model for ADC. It was found that SKBR-3, MDA-MB-453, MCF-7, and MDA-MB-468 cells had ∼800,000, ∼250,000, ∼50,000, and ∼10,000 HER2 receptors per cell, respectively. A strong linear relationship (R 2 > 0.9) was observed between HER2 receptor count and released MMAE exposure inside the cancer cells. There was an inverse relationship found between HER2 expression level and internalization rate, and cathepsin B and multidrug resistance protein 1 (MDR1) expression level varied slightly among the cell lines. The PK model was able to simultaneously capture all the PK profiles reasonably well while estimating only two parameters. Our results demonstrate a strong quantitative relationship between antigen expression level and intracellular exposure of ADCs in cancer cells. SIGNIFICANCE STATEMENT: In this manuscript, we have demonstrated a strong linear relationship between target expression level and antibody-drug conjugate (ADC) exposure inside cancer cells. We have also shown that this relationship can be accurately captured using the cell-level systems pharmacokinetics model developed for ADCs. Our results indirectly suggest that the lack of relationship between target expression and efficacy of ADC may stem from differences in the pharmacodynamic properties of cancer cells.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
-
- Ait-Oudhia S, Zhang W, Mager DE. (2017) A mechanism-based PK/PD model for hematological toxicities induced by antibody-drug conjugates. AAPS J 19:1436–1448. - PubMed
-
- Beck A, Goetsch L, Dumontet C, Corvaïa N. (2017) Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 16:315–337. - PubMed
-
- Caculitan NG, Dela Cruz Chuh J, Ma Y, Zhang D, Kozak KR, Liu Y, Pillow TH, Sadowsky J, Cheung TK, Phung Q, et al. (2017) Cathepsin B is dispensable for cellular processing of cathepsin B-cleavable antibody-drug conjugates. Cancer Res 77:7027–7037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

