Inactivation Efficacies and Mechanisms of Gas Plasma and Plasma-Activated Water against Aspergillus flavus Spores and Biofilms: a Comparative Study
- PMID: 32086309
- PMCID: PMC7170485
- DOI: 10.1128/AEM.02619-19
Inactivation Efficacies and Mechanisms of Gas Plasma and Plasma-Activated Water against Aspergillus flavus Spores and Biofilms: a Comparative Study
Abstract
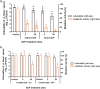
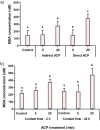
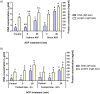
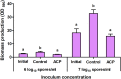
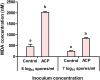
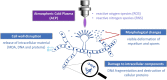
Atmospheric cold plasma (ACP) treatment is an emerging food technology for product safety and quality retention, shelf-life extension, and sustainable processing. The activated chemical species of ACP can act rapidly against microorganisms without leaving chemical residues on food surfaces. The main objectives of this study were to investigate the efficiency and mechanisms of inactivation of fungal spores and biofilms by ACP and to understand the effects of the gas-mediated and liquid-mediated modes of application against important fungal contaminants. Aspergillus flavus was selected as the model microorganism. A. flavus spores were exposed to either gas plasma (GP) or plasma-activated water (PAW), whereas gas plasma alone was used to treat A. flavus biofilms. This study demonstrated that both GP and PAW treatments independently resulted in significant decreases of A. flavus metabolic activity and spore counts, with maximal reductions of 2.2 and 0.6 log10 units for GP and PAW, respectively. The characterization of the reactive oxygen and nitrogen species in PAW and spore suspensions indicated that the concentration of secondary reactive species was an important factor influencing the antimicrobial activity of the treatment. The biofilm study showed that GP had detrimental effects on biofilm structure; however, the initial inoculum concentration prior to biofilm formation can be a crucial factor influencing the fungicidal effects of ACP.IMPORTANCE The production of mycotoxin-free food remains a challenge in both human and animal food chains. A. flavus, a mycotoxin-producing contaminant of economically important crops, was selected as the model microorganism to investigate the efficiency and mechanisms of ACP technology against fungal contaminants of food. Our study directly compares the antifungal properties of gas plasma (GP) and plasma-activated water (PAW) against fungi as well as reporting the effects of ACP treatment on biofilms produced by A. flavus.
Keywords: Aspergillus flavus; cold plasma; grains; mycotoxin.
Copyright © 2020 American Society for Microbiology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

