HNRNPA1-induced spliceopathy in a transgenic mouse model of myotonic dystrophy
- PMID: 32086392
- PMCID: PMC7071875
- DOI: 10.1073/pnas.1907297117
HNRNPA1-induced spliceopathy in a transgenic mouse model of myotonic dystrophy
Abstract
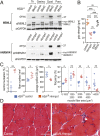
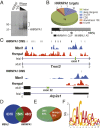
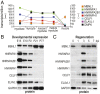
Studies on myotonic dystrophy type 1 (DM1) have led to the RNA-mediated disease model for hereditary disorders caused by noncoding microsatellite expansions. This model proposes that DM1 disease manifestations are caused by a reversion to fetal RNA processing patterns in adult tissues due to the expression of toxic CUG RNA expansions (CUGexp) leading to decreased muscleblind-like, but increased CUGBP1/ETR3-like factor 1 (CELF1), alternative splicing activities. Here, we test this model in vivo, using the mouse HSALR poly(CUG) model for DM1 and recombinant adeno-associated virus (rAAV)-mediated transduction of specific splicing factors. Surprisingly, systemic overexpression of HNRNPA1, not previously linked to DM1, also shifted DM1-relevant splicing targets to fetal isoforms, resulting in more severe muscle weakness/myopathy as early as 4 to 6 wk posttransduction, whereas rAAV controls were unaffected. Overexpression of HNRNPA1 promotes fetal exon inclusion of representative DM1-relevant splicing targets in differentiated myoblasts, and HITS-CLIP of rAAV-mycHnrnpa1-injected muscle revealed direct interactions of HNRNPA1 with these targets in vivo. Similar to CELF1, HNRNPA1 protein levels decrease during postnatal development, but are elevated in both regenerating mouse muscle and DM1 skeletal muscle. Our studies suggest that CUGexp RNA triggers abnormal expression of multiple nuclear RNA binding proteins, including CELF1 and HNRNPA1, that antagonize MBNL activity to promote fetal splicing patterns.
Keywords: CELF1; HNRNPA1; MBNL1; microsatellite; splicing.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

