Changes in kidney function among men having sex with men starting on demand tenofovir disoproxil fumarate - emtricitabine for HIV pre-exposure prophylaxis
- PMID: 32086878
- PMCID: PMC7035456
- DOI: 10.1002/jia2.25420
Changes in kidney function among men having sex with men starting on demand tenofovir disoproxil fumarate - emtricitabine for HIV pre-exposure prophylaxis
Abstract
Introduction: Daily pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) is associated with a small but statistically significant decrease in estimated glomerular filtration rate (eGFR). We assessed the renal safety of on-demand PrEP with TDF/FTC in HIV-1 uninfected men.
Methods: We used data from the randomized double-blind placebo-controlled ANRS-IPERGAY trial and its open-label extension conducted between February 2012 and June 2016 among HIV-uninfected MSM starting on-demand PrEP. Using linear mixed model, we evaluated the mean eGFR decline from baseline over time and determined risks factors associated with eGFR decline during the study.
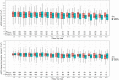
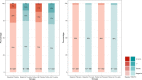
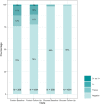
Results: During the blind phase, with a median follow-up of 9.4 months, the mean decline slope of eGFR from baseline was -0.88 and -1.53 mL/min/1.73 m2 per year in the placebo (n = 201) and the TDF/FTC group (n = 198) respectively, with a slope difference of 0.65 mL/min/1.73 m2 per year (p = 0.27). Including both phases, 389 participants started on-demand TDF/FTC with a median follow-up of 19.2 months and a mean decline of eGFR from baseline of -1.14 mL/min/1.73 m2 per year (p < 0.001). The slope of eGFR reduction was not significantly different in participants with baseline eGFR ≤ 90 mL/min/1.73 m2 (p = 0.44), age >40 years (p = 0.24) or hypertension (p = 0.21). There was a dose-response relationship between recent tenofovir exposure and lower eGFR when considering the number of pills taken in the two months prior the visit (eGFR difference of -0.88 mL/min/1.73 m2 between >15 pills/month vs. ≤15 pills/month, p < 0.01) or plasma tenofovir concentrations at the visit (eGFR difference compared to ≤2 ng/mL: >2 to ≤10ng/mL: -0.98 mL/min/1.73 m2 , >10 to ≤40ng/mL: -1.28 mL/min/1.73 m2 , >40 ng/mL: -1.82 mL/min/1.73 m2 , p < 0.001). Three participants discontinued TDF/FTC for eGFR < 60 mL/min/1.73 m2 during the OLE phase. No case of Fanconi syndrome was reported.
Conclusions: The renal safety of on-demand PrEP with TDF/FTC was good. The overall reduction and intermittent exposure to TDF/FTC may explain this good renal safety.
Keywords: HIV; PrEP; eGFR; intermittent; kidney; on-demand; tenofovir.
© 2020 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures

References
-
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet Lond Engl. 2013;381:2083–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

