Uptake, engagement, and adherence to pre-exposure prophylaxis offered after population HIV testing in rural Kenya and Uganda: 72-week interim analysis of observational data from the SEARCH study
- PMID: 32087152
- PMCID: PMC7208546
- DOI: 10.1016/S2352-3018(19)30433-3
Uptake, engagement, and adherence to pre-exposure prophylaxis offered after population HIV testing in rural Kenya and Uganda: 72-week interim analysis of observational data from the SEARCH study
Abstract
Background: Optimal strategies for pre-exposure prophylaxis (PrEP) engagement in generalised HIV epidemics are unknown. We aimed to assess PrEP uptake and engagement after population-level HIV testing and universal PrEP access to characterise gaps in the PrEP cascade in rural Kenya and Uganda.
Methods: We did a 72-week interim analysis of observational data from the ongoing SEARCH (Sustainable East Africa Research in Community Health) study. Following community sensitisation and PrEP education, we did HIV testing and offered PrEP at health fairs and facilities in 16 rural communities in western Kenya, eastern Uganda, and western Uganda. We provided enhanced PrEP counselling to individuals 15 years and older who were assessed as having an elevated HIV risk on the basis of serodifferent partnership or empirical risk score, or who otherwise self-identified as being at high risk but were not in serodifferent partnerships or identified by the risk score. PrEP follow-up visits were done at facilities, homes, or community locations. We assessed PrEP uptake within 90 days of HIV testing, programme engagement (follow-up visit attendance at week 4, week 12, and every 12 weeks thereafter), refills, self-reported adherence up to 72 weeks, and concentrations of tenofovir in hair samples from individuals reporting HIV risk and adherence during follow-up, and analysed factors associated with uptake and adherence. This study is registered with ClinicalTrials.gov, NCT01864603.
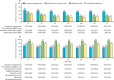
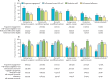
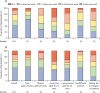
Findings: Between June 6, 2016, and June 23, 2017, 70 379 community residents 15 years or older who had not previously been diagnosed with HIV were tested during population-level HIV testing. Of these individuals, 69 121 tested HIV-negative, 12 935 of whom had elevated HIV risk (1353 [10%] serodifferent partnership, 6938 [54%] risk score, 4644 [36%] otherwise self-identified risk). 3489 (27%) initiated PrEP, 2865 (82%) of whom did so on the same day as HIV testing and 1733 (50%) of whom were men. PrEP uptake was lower among individuals aged 15-24 years (adjusted odds ratio 0·55, 95% CI 0·45-0·68) and mobile individuals (0·61, 0·41-0·91). At week 4, among 3466 individuals who initiated PrEP and did not withdraw or die before the first visit, 2215 (64%) were engaged in the programme, 1701 (49%) received medication refills, and 1388 (40%) self-reported adherence. At week 72, 1832 (56%) of 3274 were engaged, 1070 (33%) received a refill, and 900 (27%) self-reported adherence. Among participants reporting HIV risk at weeks 4-72, refills (89-93%) and self-reported adherence (70-76%) were high. Among sampled participants self-reporting adherence at week 24, the proportion with tenofovir concentrations in the hair reflecting at least four doses taken per week was 66%, and reflecting seven doses per week was 44%. Participants who stopped PrEP accepted HIV testing at 4274 (83%) of 5140 subsequent visits; half of these participants later restarted PrEP. 29 participants of 3489 who initiated PrEP had serious adverse events, including seven deaths. Five adverse events (all grade 3) were assessed as being possibly related to the study drug.
Interpretation: During population-level HIV testing, inclusive risk assessment (combining serodifferent partnership, an empirical risk score, and self-identification of HIV risk) was feasible and identified individuals who could benefit from PrEP. The biggest gap in the PrEP cascade was PrEP uptake, particularly for young and mobile individuals. Participants who initiated PrEP and had perceived HIV risk during follow-up reported taking PrEP, but one-third had drug concentrations consistent with poor adherence, highlighting the need for novel approaches and long-acting formulations as PrEP roll-out expands.
Funding: National Institutes of Health, President's Emergency Plan for AIDS Relief, Bill & Melinda Gates Foundation, and Gilead Sciences.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Lessons on PrEP from the SEARCH study in east Africa.Lancet HIV. 2020 Apr;7(4):e219-e220. doi: 10.1016/S2352-3018(20)30003-5. Epub 2020 Feb 19. Lancet HIV. 2020. PMID: 32087151 No abstract available.
References
-
- UNAIDS . Joint United Nations Programme on HIV/AIDS; Geneva: 2018. Miles to go: closing gaps, breaking barriers, righting injustices.
-
- Grulich AE, Guy R, Amin J. Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. Lancet HIV. 2018;5:e629–e637. - PubMed
-
- WHO . 2nd edn. World Health Organization; Geneva: 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

