IRF-1 mediates the suppressive effects of mTOR inhibition on arterial endothelium
- PMID: 32087218
- PMCID: PMC7138750
- DOI: 10.1016/j.yjmcc.2020.02.006
IRF-1 mediates the suppressive effects of mTOR inhibition on arterial endothelium
Abstract
Aims: Mammalian target of rapamycin (mTOR) inhibitors used in drug-eluting stents (DES) to control restenosis have been found to delay endothelialization and increase incidence of late-stent thrombosis through mechanisms not completely understood. We revealed that mTOR inhibition (mTORi) upregulated the expression of cell growth suppressor IRF-1 in primary human arterial endothelial cells (HAEC). This study aimed to examine how mTOR-regulated IRF-1 expression contributes to the suppressive effect of mTORi on arterial endothelial proliferation.
Methods and results: Western blotting, quantitative PCR, and a dual-luciferase reporter assay indicated that mTOR inhibitors rapamycin and torin 1 upregulated IRF-1 expression and increased its transcriptional activity. IRF-1 in turn contributed to the suppressive effect of mTORi by mediating HAEC apoptosis and cell cycle arrest in part through upregulation of caspase 1 and downregulation of cyclin D3, as revealed by CCK-8 assay, Annexin V binding assay, measurement of activated caspase 3, BrdU incorporation assay, and matrigel tube formation assay. In a mouse model of femoral artery wire injury, administration of rapamycin inhibited EC recovery, an effect alleviated by EC deficiency of IRF-1. Chromatin immunoprecipitation assay with HAEC and rescue expression of wild type or dominant-negative IRF-1 in EC isolated from Irf1-/- mice confirmed transcriptional regulation of IRF-1 on the expression of CASP1 and CCND3. Furthermore, mTORi activated multiple PKC members, among which PKCζ was responsible for the growth-inhibitory effect on HAEC. Activated PKCζ increased IRF1 transcription through JAK/STAT-1 and NF-κB signaling. Finally, overexpression of wild type or mutant raptor incapable of binding mTOR indicated that mTOR-free raptor contributed to PKCζ activation in mTOR-inhibited HAEC.
Conclusions: The study reveals an IRF-1-mediated mechanism that contributes to the suppressive effects of mTORi on HAEC proliferation. Further study may facilitate the development of effective strategies to reduce the side effects of DES used in coronary interventions.
Keywords: Cell proliferation; Drug-eluting stents; Endothelium; Transcription factor; mTOR.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures





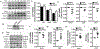
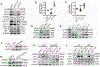
References
-
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL, Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials, The American journal of medicine 119(12) (2006) 1056–61. - PubMed
-
- Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, et al., Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents, The New England journal of medicine 356(10) (2007) 1030–9. - PubMed
-
- Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R, One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis, JACC. Cardiovascular imaging 2(5) (2009) 625–8. - PubMed
-
- Vezina C, Kudelski A, Sehgal SN, Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle, The Journal of antibiotics 28(10) (1975) 721–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

