The SMARTER Trial: Design of a trial testing tailored mHealth feedback to impact self-monitoring of diet, physical activity, and weight
- PMID: 32087342
- PMCID: PMC7269678
- DOI: 10.1016/j.cct.2020.105958
The SMARTER Trial: Design of a trial testing tailored mHealth feedback to impact self-monitoring of diet, physical activity, and weight
Abstract
Background: Self-monitoring food intake and physical activity (PA) is positively related to weight loss and the addition of feedback (FB) messages has been shown to reinforce behavior change. Moreover, the more immediate the delivery of reinforcing FB messages, the more likely they will promote the desired behaviors.
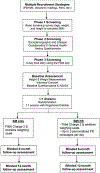
Purpose: Describe design and rationale of SMARTER, a National Institute of Heart, Lung, and Blood (NHLBI)-sponsored randomized, controlled trial, which compares the differential efficacy of two weight loss treatments among 530 adults, ages 18 and older.
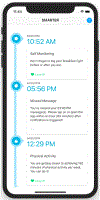
Methods: Single-site, 2-group design trial with subjects randomized 1:1 to either: 1) self-monitoring (SM), where participants self-monitor diet, PA, and weight using a commercial smartphone application (app); or 2) SM + FB, where participants self-monitor and receive real-time, tailored feedback (FB) as pop-up messages up to 3 times/day for 12 months. Daily FB messages address diet and PA behaviors and a weekly FB message addresses self-weighing. We hypothesize that subjects assigned to SM + FB will show greater weight loss at 6 and 12 months and greater sustained engagement in the program than the SM group, measured by adherence to the study's lifestyle and SM protocol. We will explore temporal relationships of the frequency, timing, and type of FB delivered and subsequent lifestyle behaviors through examination of serially collected real-time SM (diet, PA, weight) data over 12 months.
Conclusions: If efficacious, this fully scalable intervention could be efficiently translated and disseminated to reach large numbers of individuals through commercial apps at lower cost than existing in-person weight loss programs.
Keywords: Adults; Clinical trial.gov #: NCT03367936; Electronic diary; Feedback; Obesity; Physical activity tracker; Randomized controlled trial; Self-monitoring; Smartphone; Technology; Weight loss; Wi-fi scale; mHealth.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
References
-
- Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL, Machan J, STOP regain: Are there negative effects of daily weighing? J. Consult. Clin. Psychol 75 (4) (2007) 652–656. - PubMed
-
- Mokdad AH, Ford ES, Bowman BA, et al. , Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001, J. Am. Med. Assoc 289 (1) (2001) 76–79. - PubMed
-
- Digenio AG, Mancuso JP, Gerber RA, Dvorak RV, Comparison of methods for delivering a lifestyle modification program for obese patients: A randomized trial, Ann. Intern. Med 150 (4) (2009) 255–262. - PubMed
-
- Riley WT, Leveraging technology for multiple risk factor interventions, Arch. Intern. Med 172 (10) (2012) 796–798. - PubMed