OATP1A/1B, CYP3A, ABCB1, and ABCG2 limit oral availability of the NTRK inhibitor larotrectinib, while ABCB1 and ABCG2 also restrict its brain accumulation
- PMID: 32087611
- PMCID: PMC7279963
- DOI: 10.1111/bph.15034
OATP1A/1B, CYP3A, ABCB1, and ABCG2 limit oral availability of the NTRK inhibitor larotrectinib, while ABCB1 and ABCG2 also restrict its brain accumulation
Abstract
Background and purpose: Larotrectinib is a FDA-approved oral small-molecule inhibitor for treatment of neurotrophic tropomyosin receptor kinase fusion-positive cancer. We here investigated the functions of the multidrug efflux transporters ABCB1 and ABCG2, the SLCO1A/1B (OATP1A/1B) uptake transporters, and the multispecific drug-metabolizing enzyme CYP3A in larotrectinib pharmacokinetic behaviour.
Experimental approach: In vitro, transepithelial drug transport and uptake assays were performed. In vivo, larotrectinib (10 mg·kg-1 ) was administered orally to relevant genetically modified mouse models. Cell medium, plasma samples, and organ homogenates were measured by a sensitive and specific LC-MS/MS larotrectinib assay.
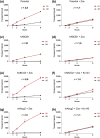
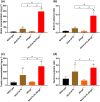
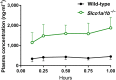
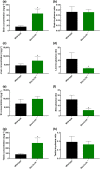
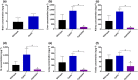
Key results: In vitro, larotrectinib was avidly transported by human (h) ABCB1 and mouse (m) Abcg2 efficiently by hABCG2 and modestly by hOATP1A2. In vivo, both mAbcb1a/1b and mAbcg2 markedly limited larotrectinib oral availability and brain and testis accumulation (by 2.1-fold, 10.4-fold, and 2.7-fold, respectively), with mAbcb1a/1b playing a more prominent role. mOatp1a/1b also restricted larotrectinib oral availability (by 3.8-fold) and overall tissue exposure, apparently by mediating substantial uptake into the liver, thus likely facilitating hepatobiliary excretion. Additionally, larotrectinib is an excellent substrate of CYP3A, which restricts the oral availability of larotrectinib and hence its tissue exposure.
Conclusions and implications: ABCG2 and especially ABCB1 limit the oral availability and brain and testis penetration of larotrectinib, while OATP1A/1B transporters restrict its systemic exposure by mediating hepatic uptake, thus allowing hepatobiliary excretion. CYP3A-mediated metabolism can strongly limit larotrectinib oral availability and hence its tissue concentrations. These insights may be useful in the further clinical development of larotrectinib.
© 2020 The British Pharmacological Society.
Conflict of interest statement
The research group of A.H.S. receives revenue from commercial distribution of some of the mouse strains used in this study. The remaining authors declare no conflicts of interest.
Figures


References
-
- Bakos, E. , Evers, R. , Calenda, G. , Tusnady, G. E. , Szakacs, G. , Varadi, A. , & Sarkadi, B. (2000). Characterization of the amino‐terminal regions in the human multidrug resistance protein (MRP1). Journal of Cell Science, 113(Pt 24), 4451–4461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

