Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson's disease patients
- PMID: 32088379
- PMCID: PMC7150647
- DOI: 10.1016/j.nbd.2020.104819
Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson's disease patients
Abstract
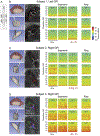
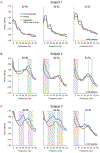
The goal of this study was to characterize the spectral characteristics and spatial topography of local field potential (LFP) activity in the internal segment of the globus pallidus (GPi) in patients with Parkinson's disease utilizing directional (segmented) deep brain stimulation (dDBS) leads. Data were collected from externalized dDBS leads of three patients with idiopathic Parkinson's disease after overnight withdrawal of parkinsonian medication at rest and during a cued reach-to-target task. Oscillatory activity across lead contacts/segments was examined in the context of lead locations and contact orientations determined using co-registered preoperative 7 Tesla (T) MRI and postoperative CT scans. Each of the three patients displayed a unique frequency spectrum of oscillatory activity in the pallidum, with prominent peaks ranging from 5 to 35 Hz, that modulated variably across subjects during volitional movement. Despite subject-specific spectral profiles, a consistent finding across patients was that oscillatory power was strongest and had the largest magnitude of modulation during movement in LFPs recorded from segments facing the postero-lateral "sensorimotor" region of GPi, whereas antero-medially-directed segmented contacts facing the internal capsule and/or anterior GPi, had relatively weaker LFP power and less modulation in the 5 to 35 Hz. In each subject, contact configurations chosen for clinically therapeutic stimulation (following data collection and blinded to physiology recordings), were in concordance with the contact pairs showing the largest amplitude of LFP oscillations in the 5-35 Hz range. Although limited to three subjects, these findings provide support for the hypothesis that the sensorimotor territory of the GPi corresponds to the site of maximal power of oscillatory activity in the 5 to 35 Hz and provides the greatest benefit in motor signs during stimulation in the GPi. Variability in oscillatory activity across patients is likely related to Parkinson's disease phenotype as well as small differences in recording location (i.e. lead location), highlighting the importance of lead location for optimizing stimulation efficacy. These data also provide compelling evidence for the use of LFP activity for the development of predictive stimulation models that may optimize patient benefits while reducing clinic time needed for programming.
Keywords: DBS; GPi; Local field potential; Parkinson's disease; Segmented.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

