Performance Status and Age as Predictors of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer
- PMID: 32089478
- PMCID: PMC7305948
- DOI: 10.1016/j.cllc.2020.01.001
Performance Status and Age as Predictors of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer
Abstract
Introduction: Immunotherapy has become a key treatment for patients with advanced non-small-cell lung cancer (NSCLC). While a survival advantage has been proven for patients who are medically fit, it is unknown whether a benefit exists for patients with poor performance status (PS).
Patients and methods: We performed a retrospective analysis of NSCLC patients who received immunotherapy in our health system. Age and PS at the time of initial immunotherapy administration were assigned based on physician documentation. Radiographic response and date of progression were assigned according to the treating physician's assessment and confirmed by the study team. Immune-related adverse events were extracted from records.
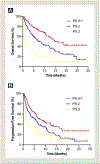
Results: We identified 285 NSCLC patients who received immunotherapy between January 2014 and April 2018. In this group, 153 patients (53.7%) had PS 0-1, 114 (40.0%) had PS 2, and 18 (6.3%) had PS 3. Response rates were similar across PS groups with 26.6% for PS 1, 25.2% for PS 2, and 23.1% for PS 3 (P = .95). Survival outcomes varied with pretreatment PS. For PS 0-1, PS 2, and PS 3, median overall survival was 14.7, 8.3, and 1.5 months (P < .001), and progression-free survival was 7.4, 5.1, and 1.3 months (P < .001). Patients aged < 70 had a lower rate (7.6%) of immune-related adverse events requiring steroids compared to patients ≥ 70 (15%) (P = .04).
Conclusion: Patients with poor baseline PS demonstrate similar response rate but inferior progression-free survival and overall survival compared to medically fit patients. Prospective trials are needed to optimize treatment for this large population.
Keywords: Elderly; ICU admissions; Immune-related adverse events; Programmed death receptor inhibitor; Steroids.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure
The authors have stated that they have no conflict of interest.
Figures
References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1epositive non–small-cell lung cancer. N Engl J Med 2016; 375:1823–33. - PubMed
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 2018; 378:2078–92. - PubMed
-
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med 2018; 379:2040–51. - PubMed
-
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378:2288–301. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous