Ralstonia solanacearum Type III Effectors with Predicted Nuclear Localization Signal Localize to Various Cell Compartments and Modulate Immune Responses in Nicotiana spp
- PMID: 32089660
- PMCID: PMC7012579
- DOI: 10.5423/PPJ.OA.08.2019.0227
Ralstonia solanacearum Type III Effectors with Predicted Nuclear Localization Signal Localize to Various Cell Compartments and Modulate Immune Responses in Nicotiana spp
Erratum in
-
Erratum: Ralstonia solanacearum Type III Effectors with Predicted Nuclear Localization Signal Localize to Various Cell Compartments and Modulate Immune Responses in Nicotiana spp.Plant Pathol J. 2020 Jun 1;36(3):303. doi: 10.5423/PPJ.ER.08.2019.0227. Plant Pathol J. 2020. PMID: 32547346 Free PMC article.
Abstract
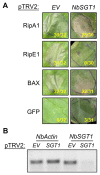
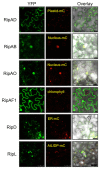
Ralstonia solanacearum (Rso) is a causal agent of bacterial wilt in Solanaceae crops worldwide including Republic of Korea. Rso virulence predominantly relies on type III secreted effectors (T3Es). However, only a handful of Rso T3Es have been characterized. In this study, we investigated subcellular localization of and manipulation of plant immunity by 8 Rso T3Es predicted to harbor a nuclear localization signal (NLS). While 2 of these T3Es elicited cell death in both Nicotiana benthamiana and N. tabacum, only one was dependent on suppressor of G2 allele of skp1 (SGT1), a molecular chaperone of nucleotide-binding and leucine-rich repeat immune receptors. We also identified T3Es that differentially regulate flg22-induced reactive oxygen species production and gene expression. Interestingly, several of the NLS-containing T3Es translationally fused with yellow fluorescent protein accumulated in subcellular compartments other than the cell nucleus. Our findings bring new clues to decipher Rso T3E function in planta.
Keywords: Nicotiana spp.; Ralstonia solanacearum; bacterial wilt; innate immunity; type III effectors.
© The Korean Society of Plant Pathology.
Figures


References
-
- Choi S, Jayaraman J, Segonzac C, Park H-J, Park H, Han S-W, Sohn KH. Pseudomonas syringae pv. actinidiae type III effectors localized at multiple cellular compartments activate or suppress innate immune responses in Nicotiana benthamiana. Front Plant Sci. 2017;8:2157. doi: 10.3389/fpls.2017.02157. - DOI - PMC - PubMed
-
- de Lange O, Schreiber T, Schandry N, Radeck J, Braun KH, Koszinowski J, Heuer H, Strauß A, Lahaye T. Breaking the DNA-binding code of Ralstonia solanacearum TAL effectors provides new possibilities to generate plant resistance genes against bacterial wilt disease. New Phytol. 2013;199:773–786. doi: 10.1111/nph.12324. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

