Comparative Analysis of Adipose-Derived Stromal Cells and Their Secretome for Auricular Cartilage Regeneration
- PMID: 32089711
- PMCID: PMC7023823
- DOI: 10.1155/2020/8595940
Comparative Analysis of Adipose-Derived Stromal Cells and Their Secretome for Auricular Cartilage Regeneration
Abstract
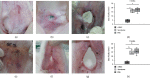
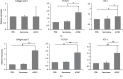
Adipose-derived stromal cells (ADSCs) can repair auricular cartilage defects. Furthermore, stem cell secretome may also be a promising biological therapeutic option, which is equal to or even superior to the stem cell. We explored the therapeutic efficacies of ADSCs and their secretome in terms of rabbit auricular cartilage regeneration. ADSCs and their secretome were placed into surgically created auricular cartilage defects. After 4 and 8 weeks, the resected auricles were histopathologically and immunohistochemically examined. We used real-time PCR to determine the levels of genes expressing collagen type II, transforming growth factor-β1 (TGF-β1), and insulin-like growth factor-1 (IGF-1). ADSCs significantly improved auricular cartilage regeneration at 4 and 8 weeks, compared to the secretome and PBS groups, as revealed by gross examination, histopathologically and immunohistochemically. ADSCs upregulated the expression of collagen type II, TGF-β1, and IGF-1 more so than did the secretome or PBS. The expression levels of collagen type II and IGF-1 were significantly higher at 8 weeks than at 4 weeks after ADSC injection. Although ADSCs thus significantly enhanced new cartilage formation, their secretome did not. Therefore, ADSCs may be more effective than their secretome in the repair of auricular cartilage defect.
Copyright © 2020 Se-Joon Oh et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Pleumeekers M. M., Nimeskern L., Koevoet W. L. M., Karperien M., Stok K. S., van Osch G. J. V. M. Cartilage regeneration in the head and neck area: combination of ear or nasal chondrocytes and mesenchymal stem cells improves cartilage production. Plastic and Reconstructive Surgery. 2015;136(6):762e–774e. doi: 10.1097/PRS.0000000000001812. - DOI - PubMed
-
- Liu T. M., Martina M., Hutmacher D. W., Hui J. H., Lee E. H., Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25(3):750–760. doi: 10.1634/stemcells.2006-0394. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

