Gap-enhanced Raman tags: fabrication, optical properties, and theranostic applications
- PMID: 32089735
- PMCID: PMC7019156
- DOI: 10.7150/thno.39968
Gap-enhanced Raman tags: fabrication, optical properties, and theranostic applications
Abstract
Gap-enhanced Raman tags (GERTs) are emerging probes of surface-enhanced Raman scattering (SERS) spectroscopy that have found promising analytical, bioimaging, and theranostic applications. Because of their internal location, Raman reporter molecules are protected from unwanted external environments and particle aggregation and demonstrate superior SERS responses owing to the strongly enhanced electromagnetic fields in the gaps between metal core-shell structures. In this review, we discuss recent progress in the synthesis, simulation, and experimental studies of the optical properties and biomedical applications of novel spherically symmetrical and anisotropic GERTs fabricated with common plasmonic metals-gold (Au) and silver (Ag). Our discussion is focused on the design and synthetic strategies that ensure the optimal parameters and highest enhancement factors of GERTs for sensing and theranostics. In particular, we consider various core-shell structures with build-in nanogaps to explain why they would benefit the plasmonic GERTs as a superior SERS tag and how this would help future research in clinical analytics and therapeutics.
Keywords: bioimaging; gap-enhanced Raman tags (GERTS); plasmonic core-shell nanoparticles; plasmonic photothermal therapy; surface-enhanced Raman scattering (SERS); theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

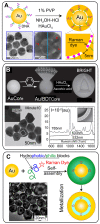

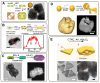

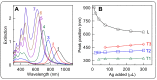
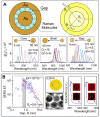





References
-
- Le Ru EC, Etchegoin PG. Single-molecule surface-enhanced Raman spectroscopy. Annu Rev Phys Chem. 2012;63:65–87. - PubMed
-
- Fleischmann M, Hendra PJ, MsQuillan AJ. Raman spectra of pyridine absorbed at a silver electrode. Chem Phys Lett. 1974;26:163–66.
-
- Jeanmarie DL, Van Duyne RP. Surface Raman spectroelectrochemistry. Part 1: Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem. 1977;84:1–20.
-
- Albrecht MG, Creighton JA. Anomalously intense Raman spectra of pyridine at a silver electrode. J Am Chem Soc. 1977;99:5215–17.
-
- Lim D-K, Jeon K-S, Hwang J-H, Kim H, Kwon S, Shu YD. et al. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat Nano. 2011;6:452–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

