Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis
- PMID: 32089744
- PMCID: PMC7019149
- DOI: 10.7150/thno.39486
Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis
Abstract
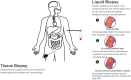
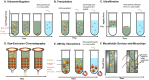
Prostate cancer (PCa) is a leading cause of cancer death for males in western countries. The current gold standard for PCa diagnosis - template needle biopsies - often does not convey a true representation of the molecular profile given sampling error and complex tumour heterogeneity. Presently available biomarker blood tests have limited accuracy. There is a growing demand for novel diagnostic approaches to reduce both the number of men with an abnormal PSA/ DRE who undergo invasive biopsy and the number of cores collected per biopsy. 'Liquid biopsy' is a minimally invasive biofluid-based approach that has the potential to provide information and improve the accuracy of diagnosis for patients' treatment selection, prognostic counselling and development of risk-adjusted follow-up protocols. Extracellular vesicles (EVs) are lipid bilayer-delimited particles released by tumour cells which may provide a real-time snapshot of the entire tumour in a non-invasive way. EVs can regulate physiological processes and mediate systemic dissemination of various types of cancers. Emerging evidence suggests that EVs have crucial roles in PCa development and metastasis. Most importantly, EVs are directly derived from their parent cells with their information. EVs contain components including proteins, mRNAs, DNA fragments, non-coding RNAs and lipids, and play a critical role in intercellular communication. Therefore, EVs hold promise for the discovery of liquid biopsy-based biomarkers for PCa diagnosis. Here, we review the current approaches for EV isolation and analysis, summarise the recent advances in EV protein biomarkers in PCa and focus on liquid biopsy-based EV biomarkers in PCa diagnosis for personalised medicine.
Keywords: biomarker; diagnosis; extracellular vesicles; liquid biopsy; prostate cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

