Cordyceps cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF- κ B/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice
- PMID: 32089779
- PMCID: PMC7026739
- DOI: 10.1155/2020/7912763
Cordyceps cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF- κ B/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice
Abstract
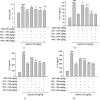
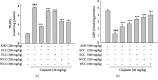
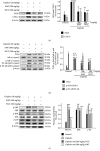
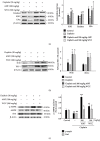
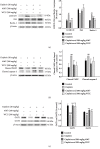
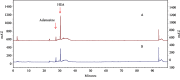
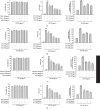
Acute kidney injury (AKI) is a common clinical problem, characterized by a sudden loss of renal function, a high risk of death, and the eventual development of renal fibrosis and renal failure. Cordyceps cicadae is a traditional Chinese medicine with the potential function of kidney protection. We analyze two sputum extracts, a water extract (WCC), and an ethanol extract (ECC), to assess the potential of treating AKI in an animal model of kidney injury induced by cisplatin. A nephrotoxic mouse model was first established by intraperitoneal injection of cisplatin. Subsequently, WCC and ECC were orally administered in these mice. The results show that WCC and ECC significantly alleviated cisplatin-induced AKI renal histological changes, serum creatinine (CRE) and blood urea nitrogen (BUN) production, and the levels of NO, TNF-α, IL-1β, and IL-6. The levels of malondialdehyde (MDA) and glutathione (GSH) were suppressed by administration of WCC and ECC. However, WCC treatment prevented these changes significantly better than ECC treatment. In addition, Western blot data showed that WCC attenuated the cisplatin-induced protein expression of cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS), as well as inhibiting nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) activation in the kidney tissues. Furthermore, WCC greatly inhibited the expression of Toll-like receptor 4 (TLR4) and cisplatin-induced NF-κB activation, as well as dramatically increasing the production of antioxidative enzymes (i.e., superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase 1 (HO-1)), silent information regulator T1 (Sirt1), and p-AMP-activated protein kinase (AMPK) in the kidney tissues. In addition, we found that WCC increased the expression levels of the autophagy-related proteins LC3B and Beclin-1; proapoptotic proteins, including cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) 1; and organic anion transporters 1 (OAT1) and 3 (OAT3) in the kidney tissues. Finally, WCC, ECC, and two bioactive compounds-adenosine and N6-(2-hydroxyethyl) adenosine (HEA)-inhibited the production of nitrite oxide (NO) and intracellular reactive oxygen species (ROS) triggered by lipopolysaccharide- (LPS-) stimulated RAW264.7 macrophages in vitro. Collectively, WCC could provide a potential therapeutic candidate for the prevention of cisplatin-induced kidney injury through the inhibition of oxidative stress and inflammation.
Copyright © 2020 Jeng-Shyan Deng et al.
Conflict of interest statement
All authors have no conflicts of interests.
Figures


References
-
- Huang T. H., Wu T. H., Guo Y. H., Li T. L., Chan Y. L., Wu C. J. The concurrent treatment of Scutellaria baicalensis Georgi enhances the therapeutic efficacy of cisplatin but also attenuates chemotherapy-induced cachexia and acute kidney injury. Journal of Ethnopharmacology. 2019;243, article 112075 doi: 10.1016/j.jep.2019.112075. - DOI - PubMed
-
- Qi Z., Li Z., Li W., et al. Pseudoginsengenin DQ exhibits therapeutic effects in cisplatin-induced acute kidney injury via sirt1/NF-κB and caspase signaling pathway without compromising its antitumor activity in mice. Molecules. 2018;23(11):p. 3038. doi: 10.3390/molecules23113038. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

