Putative autoantibodies in the cerebrospinal fluid of Alzheimer's disease patients
- PMID: 32089828
- PMCID: PMC7008601
- DOI: 10.12688/f1000research.21140.1
Putative autoantibodies in the cerebrospinal fluid of Alzheimer's disease patients
Abstract
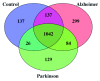
Background: Recent efforts have described an immunogenic component to the pathobiology of Alzheimer's disease (AD) and Parkinson's disease (PD). However, current methods of studying fluid autoantibodies, such as enzyme-linked immunosorbent assays and immunohistochemistry, are hypothesis-driven and not optimal for discovering new autoantibody biomarkers by proteome-wide screening. Recently, we developed a general mass spectrometry-based approach to identify tissue-specific autoantibodies in serum, at a proteome-wide level. In this study, we adapted the method to explore novel autoantibody biomarkers in the cerebrospinal fluid (CSF) of AD and PD patients. Methods: CSF samples were obtained from 10 headache control individuals, 10 AD patients and 10 PD patients. Antibodies present in the CSF were isolated by immobilization to protein-G magnetic beads. These antibodies were incubated with a brain tissue extract, prepared from frontal cortex, pons, cerebellum and brain stem. Protein antigens captured by the protein-G magnetic bead-bound antibodies were digested with trypsin and analyzed using mass spectrometry. Autoantibody candidates were selected by 1) detection in one or less individuals of the control group and 2) identification in at least half of the patient groups. Results: There were 16 putative autoantibody biomarkers selected from the AD group. Glia-derived nexin autoantibody was detected in eight of ten AD patients and was absent in the control group. Other AD pathology-related targets were also identified, such as actin-interaction protein, quinone oxidoreductase, sushi repeat-containing protein, metalloproteinase inhibitor 2, IP3 receptor 1 and sarcoplasmic/endoplasmic reticulum calcium ATPase 2. An additional eleven autoantibody targets were also identified in the present experiment, although their link to AD is not clear. No autoantibodies in the PD group satisfied our selection criteria. Conclusion: Our unbiased mass spectrometry method was able to detect new putative CSF autoantibody biomarkers of AD. Further investigation into the involvement of humoral autoimmunity in AD and PD pathobiology may be warranted.
Keywords: 4; 5-triphosphate receptor type 1; Alzheimer’s disease; Parkinson’s disease; actin-interacting protein; autoantibodies; biomarkers; cerebrospinal fluid; glia-derived nexin; immuno-mass spectrometry; inositol 1; metalloproteinase inhibitor 2; quinone oxidoreductase; sarco/endoplasmic reticulum calcium ATPase 2; sushi repeat-containing protein.
Copyright: © 2019 Lim B et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
Similar articles
-
Improved Differential Diagnosis of Alzheimer's Disease by Integrating ELISA and Mass Spectrometry-Based Cerebrospinal Fluid Biomarkers.J Alzheimers Dis. 2019;67(2):639-651. doi: 10.3233/JAD-180855. J Alzheimers Dis. 2019. PMID: 30614806 Free PMC article.
-
Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases.Eur J Neurosci. 2016 Jan;43(1):3-16. doi: 10.1111/ejn.13056. Epub 2015 Oct 15. Eur J Neurosci. 2016. PMID: 26332448 Free PMC article.
-
Autoantibodies Toward the Angiotensin 2 Type 1 Receptor: A Novel Autoantibody in Alzheimer's Disease.J Alzheimers Dis. 2015;47(2):523-9. doi: 10.3233/JAD-150053. J Alzheimers Dis. 2015. PMID: 26401573
-
Cerebrospinal Fluid Biomarkers for Target Engagement and Efficacy in Clinical Trials for Alzheimer's and Parkinson's Diseases.Front Neurol Neurosci. 2016;39:117-23. doi: 10.1159/000445452. Epub 2016 Jul 26. Front Neurol Neurosci. 2016. PMID: 27463974 Review.
-
Cerebrospinal fluid biomarkers in Alzheimer's and Parkinson's diseases-From pathophysiology to clinical practice.Mov Disord. 2016 Jun;31(6):836-47. doi: 10.1002/mds.26656. Epub 2016 May 4. Mov Disord. 2016. PMID: 27145480 Review.
Cited by
-
The plasma peptides of Alzheimer's disease.Clin Proteomics. 2021 Jun 28;18(1):17. doi: 10.1186/s12014-021-09320-2. Clin Proteomics. 2021. PMID: 34182925 Free PMC article.
-
B cell profiles, antibody repertoire and reactivity reveal dysregulated responses with autoimmune features in melanoma.Nat Commun. 2023 Jun 8;14(1):3378. doi: 10.1038/s41467-023-39042-y. Nat Commun. 2023. PMID: 37291228 Free PMC article.
-
The Functional Roles and Applications of Immunoglobulins in Neurodegenerative Disease.Int J Mol Sci. 2020 Jul 26;21(15):5295. doi: 10.3390/ijms21155295. Int J Mol Sci. 2020. PMID: 32722559 Free PMC article. Review.
-
"Let Food Be Thy Medicine": Gluten and Potential Role in Neurodegeneration.Cells. 2021 Mar 30;10(4):756. doi: 10.3390/cells10040756. Cells. 2021. PMID: 33808124 Free PMC article. Review.
-
The role of autoantibodies in Alzheimer's disease: Pathogenetic connections or epiphenomena?Alzheimers Dement. 2025 Jul;21(7):e70484. doi: 10.1002/alz.70484. Alzheimers Dement. 2025. PMID: 40696840 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

