Discovery of novel 1,4-disubstituted 1,2,3-triazole phenylalanine derivatives as HIV-1 capsid inhibitors
- PMID: 32089839
- PMCID: PMC7034945
- DOI: 10.1039/c9ra05869a
Discovery of novel 1,4-disubstituted 1,2,3-triazole phenylalanine derivatives as HIV-1 capsid inhibitors
Abstract
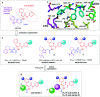
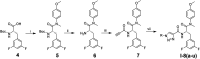
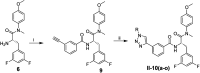
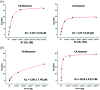
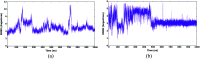
The HIV-1 capsid (CA) protein plays crucial roles in both early and late stages of the viral life cycle, which has intrigued researchers to target it to develop anti-HIV drugs. Accordingly, in this research, we report the design, synthesis and biological evaluation of a series of novel phenylalanine derivatives as HIV-1 CA protein inhibitors using the Cu(I)-catalyzed azide and alkyne 1,3-dipolar cycloaddition (CuAAC) reaction. Among this series of inhibitors, compound II-10c displayed a remarkable anti-HIV activity (EC50 = 2.13 μM, CC50 > 35.49 μM). Furthermore, surface plasmon resonance (SPR) binding assays showed that compounds II-10c and PF-74 (lead compound) have similar affinities to HIV-1 CA monomer. Further investigation showed that the weak permeability and water solubility of representative compounds were probably the important factors that restricted their cell-based activity. Preliminary structure-activity relationships (SARs) were inferred based on the activities of these compounds, and their known structure. The most promising new compound was studied with molecular dynamics simulation (MD) to determine the preferred interactions with the drug target. Finally, the activities of members of this series of inhibitors were deeply inspected to find the potential reasons for their anti-HIV-1 activity from various perspectives. This highlights the important factors required to design compounds with improved potency.
Keywords: CuAAC; HIV-1; HIV-1 capsid protein; Molecular dynamics simulation; Phenylalanine derivatives; SAR; Surface plasmon resonance.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures
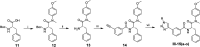
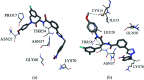
Similar articles
-
Discovery of phenylalanine derivatives as potent HIV-1 capsid inhibitors from click chemistry-based compound library.Eur J Med Chem. 2018 Oct 5;158:478-492. doi: 10.1016/j.ejmech.2018.09.029. Epub 2018 Sep 11. Eur J Med Chem. 2018. PMID: 30243152 Free PMC article.
-
Design, Synthesis and Structure-Activity Relationships of Phenylalanine-Containing Peptidomimetics as Novel HIV-1 Capsid Binders Based on Ugi Four-Component Reaction.Molecules. 2022 Sep 14;27(18):5995. doi: 10.3390/molecules27185995. Molecules. 2022. PMID: 36144727 Free PMC article.
-
Design, synthesis and structure-activity relationships of 4-phenyl-1H-1,2,3-triazole phenylalanine derivatives as novel HIV-1 capsid inhibitors with promising antiviral activities.Eur J Med Chem. 2020 Mar 15;190:112085. doi: 10.1016/j.ejmech.2020.112085. Epub 2020 Jan 24. Eur J Med Chem. 2020. PMID: 32066010 Free PMC article.
-
Discovery of novel anti-HIV agents via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry-based approach.Expert Opin Drug Discov. 2016 Sep;11(9):857-71. doi: 10.1080/17460441.2016.1210125. Epub 2016 Jul 21. Expert Opin Drug Discov. 2016. PMID: 27400283 Review.
-
Recent Progress in 1H-1,2,3-triazoles as Potential Antifungal Agents.Curr Top Med Chem. 2021;21(23):2109-2133. doi: 10.2174/1568026621666210913122828. Curr Top Med Chem. 2021. PMID: 34517801 Review.
Cited by
-
The Synthesis of Triazolium Salts as Antifungal Agents: A Biological and In Silico Evaluation.Antibiotics (Basel). 2022 Apr 27;11(5):588. doi: 10.3390/antibiotics11050588. Antibiotics (Basel). 2022. PMID: 35625232 Free PMC article.
-
Antibacterial effects of quercetagetin are significantly enhanced upon conjugation with chitosan engineered copper oxide nanoparticles.Biometals. 2024 Feb;37(1):171-184. doi: 10.1007/s10534-023-00539-0. Epub 2023 Oct 4. Biometals. 2024. PMID: 37792257
-
Separation performance of the calix[8]arene functionalized with polyethylene glycol units for capillary gas chromatography.Anal Sci. 2023 Jun;39(6):989-998. doi: 10.1007/s44211-023-00307-7. Epub 2023 Feb 24. Anal Sci. 2023. PMID: 36826712
-
Design, Synthesis, and Mechanism Study of Benzenesulfonamide-Containing Phenylalanine Derivatives as Novel HIV-1 Capsid Inhibitors with Improved Antiviral Activities.J Med Chem. 2020 May 14;63(9):4790-4810. doi: 10.1021/acs.jmedchem.0c00015. Epub 2020 Apr 29. J Med Chem. 2020. PMID: 32298111 Free PMC article.
-
Recent Advances in HIV-1 Gag Inhibitor Design and Development.Molecules. 2020 Apr 7;25(7):1687. doi: 10.3390/molecules25071687. Molecules. 2020. PMID: 32272714 Free PMC article. Review.
References
-
- Song Y. N. Zhan P. Li X. Rai D. De Clercq E. Liu X. Y. Curr. Med. Chem. 2013;20:815–832. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

