Increased Temperature Facilitates Adeno-Associated Virus Vector Transduction of Colorectal Cancer Cell Lines in a Manner Dependent on Heat Shock Protein Signature
- PMID: 32090115
- PMCID: PMC7031720
- DOI: 10.1155/2020/9107140
Increased Temperature Facilitates Adeno-Associated Virus Vector Transduction of Colorectal Cancer Cell Lines in a Manner Dependent on Heat Shock Protein Signature
Abstract
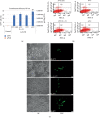
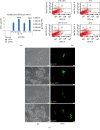
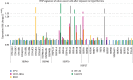
Colorectal cancer (CRC) is one of the most common cancers in human population. A great achievement in the treatment of CRC was the introduction of targeted biological drugs and solutions of chemotherapy, combined with hyperthermia. Cytoreductive surgery and HIPEC (hyperthermic intraperitoneal chemotherapy) extends the patients' survival with CRC. Recently, gene therapy approaches are also postulated. The studies indicate the possibility of enhancing the gene transfer to cells by recombinant adeno-associated vectors (rAAV) at hyperthermia. The rAAV vectors arouse a lot of attention in the field of cancer treatment due to many advantages. In this study, the effect of elevated temperature on the transduction efficiency of rAAV vectors on CRC cells with different origin and gene profile was examined. The effect of heat shock on the penetration of rAAV vectors into CRC cells in relation with the expression of HSP and AAV receptor genes was tested. It was found that the examined cells under hyperthermia (43°C, 1 h) are transduced at a higher level than in normal conditions (37°C). The results also indicate that studied RKO, HT-29, and LS411N cell lines express HSP genes at different levels under both 37°C and 43°C. Moreover, the results showed that the expression of AAV receptors increases in response to elevated temperature. The study suggests that increased rAAV transfer to CRC can be achieved under elevated temperature conditions. The obtained results provide information relevant to the design of new solutions in CRC therapy based on the combination of hyperthermia, chemotherapy, and gene therapy.
Copyright © 2020 Alicja Bieńkowska et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication.
Figures







References
-
- Matlawska K., Kalinowska U., Erxleben A., Osiecka R., Ochocki J. Novel analogues of 5-fluorouracil–synthesis, x-ray crystallography, and cytotoxic effects in normal human peripheral blood lymphocytes and colon adenocarcinoma HT 29. European Journal of Inorganic Chemistry. 2005;2005(15):3109–3117. doi: 10.1002/ejic.200500015. - DOI
-
- Ochocki J., Andrea Erxleben A., Lippert B. Synthesis and spectroscopy of phosphonate derivatives of uracil and thymine. X-ray crystal structure of diethyl 6-uracilmethylphosphonate. Journal of Heterocyclic Chemistry. 1997;34:1179–1184. doi: 10.1002/jhet.5570340414. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

