Characterization of a reproducible model of fracture healing in mice using an open femoral osteotomy
- PMID: 32090156
- PMCID: PMC7025178
- DOI: 10.1016/j.bonr.2020.100250
Characterization of a reproducible model of fracture healing in mice using an open femoral osteotomy
Erratum in
-
Erratum regarding missing Declaration of competing interest statements in previously published articles.Bone Rep. 2021 Apr 29;14:101084. doi: 10.1016/j.bonr.2021.101084. eCollection 2021 Jun. Bone Rep. 2021. PMID: 34150955 Free PMC article.
Abstract
Purpose: The classic fracture model, described by Bonnarens and Einhorn in 1984, enlists a blunt guillotine to generate a closed fracture in a pre-stabilized rodent femur. However, in less experienced hands, this technique yields considerable variability in fracture pattern and requires highly-specialized equipment. This study describes a reproducible and low-cost model of mouse fracture healing using an open femoral osteotomy.
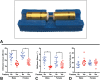
Methods: Femur fractures were produced in skeletally mature male and female mice using an open femoral osteotomy after intramedullary stabilization. Mice were recovered for up to 28 days prior to analysis with microradiographs, histomorphometry, a novel μCT methodology, and biomechanical torsion testing at weekly intervals.
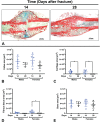
Results: Eight mice were excluded due to complications (8/193, 4.1%), including unacceptable fracture pattern (2/193, 1.0%). Microradiographs showed progression of the fracture site to mineralized callus by 14 days and remodelling 28 days after surgery. Histomorphometry from 14 to 28 days revealed decreased cartilage area and maintained bone area. μCT analysis demonstrated a reduction in mineral surface from 14 to 28 days, stable mineral volume, decreased strut number, and increased strut thickness. Torsion testing at 21 days showed that fractured femurs had 61% of the ultimate torque, 63% of the stiffness, and similar twist to failure when compared to unfractured contralateral femurs.
Conclusions: The fracture model described herein, an open femoral osteotomy, demonstrated healing comparable to that reported using closed techniques. This simple model could be used in future research with improved reliability and reduced costs compared to the current options.
Keywords: Bone repair; Fracture healing; Micro-CT fracture analysis; Mouse fracture model; Osteotomy.
© 2020 The Authors.
Figures






References
-
- Alkhiary Y.M., Gerstenfeld L.C., Krall E., Westmore M., Sato M., Mitlak B.H., Einhorn T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J. Bone Joint Surg. 2005;87(4):731–741. American volume. - PubMed
-
- Baldik Y., Diwan A.D., Appleyard R.C., Fang Z.M., Wang Y., Murrell G.A. Deletion of iNOS gene impairs mouse fracture healing. Bone. 2005;37(1):32–36. - PubMed
-
- Bonnarens F., Einhorn T.A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 1984;2(1):97–101. - PubMed
-
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010;25(7):1468–1486. - PubMed
LinkOut - more resources
Full Text Sources

