Rapid in-country sequencing of whole virus genomes to inform rabies elimination programmes
- PMID: 32090172
- PMCID: PMC7001756
- DOI: 10.12688/wellcomeopenres.15518.2
Rapid in-country sequencing of whole virus genomes to inform rabies elimination programmes
Abstract
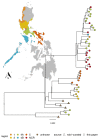
Genomic surveillance is an important aspect of contemporary disease management but has yet to be used routinely to monitor endemic disease transmission and control in low- and middle-income countries. Rabies is an almost invariably fatal viral disease that causes a large public health and economic burden in Asia and Africa, despite being entirely vaccine preventable. With policy efforts now directed towards achieving a global goal of zero dog-mediated human rabies deaths by 2030, establishing effective surveillance tools is critical. Genomic data can provide important and unique insights into rabies spread and persistence that can direct control efforts. However, capacity for genomic research in low- and middle-income countries is held back by limited laboratory infrastructure, cost, supply chains and other logistical challenges. Here we present and validate an end-to-end workflow to facilitate affordable whole genome sequencing for rabies surveillance utilising nanopore technology. We used this workflow in Kenya, Tanzania and the Philippines to generate rabies virus genomes in two to three days, reducing costs to approximately £60 per genome. This is over half the cost of metagenomic sequencing previously conducted for Tanzanian samples, which involved exporting samples to the UK and a three- to six-month lag time. Ongoing optimization of workflows are likely to reduce these costs further. We also present tools to support routine whole genome sequencing and interpretation for genomic surveillance. Moreover, combined with training workshops to empower scientists in-country, we show that local sequencing capacity can be readily established and sustainable, negating the common misperception that cutting-edge genomic research can only be conducted in high resource laboratories. More generally, we argue that the capacity to harness genomic data is a game-changer for endemic disease surveillance and should precipitate a new wave of researchers from low- and middle-income countries.
Keywords: MinION; dog-mediated rabies; field sequencing; lyssavirus; nanopore; neglected tropical diseases; phylogenetic; rabies virus; surveillance; whole genome sequencing; zoonoses.
Copyright: © 2020 Brunker K et al.
Conflict of interest statement
No competing interests were disclosed.
Figures






References
-
- Abela-Ridder B: Laboratory techniques in rabies, fifth edition. Volume 2. Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO. (Rupprecht CE, Fooks AR, Abela-Ridder B: Eds.) (5th ed., Vol. 2). Geneva: Geneva: World Health Organization.2018. Reference Source
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

