Molecular mechanisms of cold pain
- PMID: 32090187
- PMCID: PMC7025288
- DOI: 10.1016/j.ynpai.2020.100044
Molecular mechanisms of cold pain
Abstract
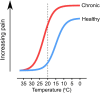
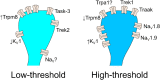
The sensation of cooling is essential for survival. Extreme cold is a noxious stimulus that drives protective behaviour and that we thus perceive as pain. However, chronic pain patients suffering from cold allodynia paradoxically experience innocuous cooling as excruciating pain. Peripheral sensory neurons that detect decreasing temperature express numerous cold-sensitive and voltage-gated ion channels that govern their response to cooling in health and disease. In this review, we discuss how these ion channels control the sense of cooling and cold pain under physiological conditions, before focusing on the molecular mechanisms by which ion channels can trigger pathological cold pain. With the ever-rising number of patients burdened by chronic pain, we end by highlighting the pressing need to define the cells and molecules involved in cold allodynia and so identify new, rational drug targets for the analgesic treatment of cold pain.
Keywords: Cold; Cold allodynia; Dorsal root ganglion; Ion channels; Neuropathic pain; Nociception; Pain; Thermosensation.
© 2020 Published by Elsevier Inc.
Figures

References
-
- Abrahamsen B., Zhao J., Asante C.O., Cendan C.M., Marsh S., Martinez-Barbera J.P., Nassar M.A., Dickenson A.H., Wood J.N. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Akopian A.N., Souslova V., England S., Okuse K., Ogata N., Ure J., Smith A., Kerr B.J., McMahon S.B., Boyce S., Hill R., Stanfa L.C., Dickenson A.H., Wood J.N. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 1999;2:541–548. - PubMed
-
- Amaya F., Wang H., Costigan M., Allchorne A.J., Hatcher J.P., Egerton J., Stean T., Morisset V., Grose D., Gunthorpe M.J., Chessell I.P., Tate S., Green P.J., Woolf C.J. The voltage-gated sodium channel nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 2006;26:12852–12860. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

