Carbon substrate re-orders relative growth of a bacterium using Mo-, V-, or Fe-nitrogenase for nitrogen fixation
- PMID: 32090445
- PMCID: PMC7187303
- DOI: 10.1111/1462-2920.14955
Carbon substrate re-orders relative growth of a bacterium using Mo-, V-, or Fe-nitrogenase for nitrogen fixation
Erratum in
-
Carbon substrate re-orders relative growth of a bacterium using Mo-, V-, or Fe-nitrogenase for nitrogen fixation.Environ Microbiol. 2022 Apr;24(4):2170-2176. doi: 10.1111/1462-2920.16001. Environ Microbiol. 2022. PMID: 35478483 Free PMC article. No abstract available.
Abstract
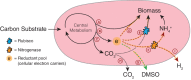
Biological nitrogen fixation is catalyzed by the molybdenum (Mo), vanadium (V) and iron (Fe)-only nitrogenase metalloenzymes. Studies with purified enzymes have found that the 'alternative' V- and Fe-nitrogenases generally reduce N2 more slowly and produce more byproduct H2 than the Mo-nitrogenase, leading to an assumption that their usage results in slower growth. Here we show that, in the metabolically versatile photoheterotroph Rhodopseudomonas palustris, the type of carbon substrate influences the relative rates of diazotrophic growth based on different nitrogenase isoforms. The V-nitrogenase supports growth as fast as the Mo-nitrogenase on acetate but not on the more oxidized substrate succinate. Our data suggest that this is due to insufficient electron flux to the V-nitrogenase isoform on succinate compared with acetate. Despite slightly faster growth based on the V-nitrogenase on acetate, the wild-type strain uses exclusively the Mo-nitrogenase on both carbon substrates. Notably, the differences in H2 :N2 stoichiometry by alternative nitrogenases (~1.5 for V-nitrogenase, ~4-7 for Fe-nitrogenase) and Mo-nitrogenase (~1) measured here are lower than prior in vitro estimates. These results indicate that the metabolic costs of V-based nitrogen fixation could be less significant for growth than previously assumed, helping explain why alternative nitrogenase genes persist in diverse diazotroph lineages and are broadly distributed in the environment.
© 2020 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures



References
-
- Andersen, K. , and Shanmugam, K.T. (1977) Energetics of biological nitrogen fixation: determination of ratio of formation of H2 to NH4 + catalyzed by nitrogenase of <styled-content style="fixed-case">Klebsiella pneumoniae</styled-content> in vivo. J Gen Microbiol 103: 107–122. - PubMed
-
- Bellenger, J.‐P. , Wichard, T. , Xu, Y. , and Kraepiel, A.M.L. (2011) Essential metals for nitrogen fixation in a free‐living N2‐fixing bacterium: chelation, homeostasis and high use efficiency. Environ Microbiol 13: 1395–1411. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

