Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial
- PMID: 32090517
- PMCID: PMC7065111
- DOI: 10.1002/oby.22726
Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial
Abstract
Objective: Previous studies have shown additive weight loss when intensive behavioral therapy (IBT) was combined with weight-loss medication. The present multisite study provides the first evaluation, in primary care, of the effect of the Centers for Medicare and Medicaid Services-based IBT benefit, delivered alone (with placebo) or in combination with liraglutide 3.0 mg.
Methods: The Satiety and Clinical Adiposity-Liraglutide Evidence in individuals with and without diabetes (SCALE) IBT was a 56-week, randomized, double-blind, placebo-controlled, multicenter trial in individuals with obesity who received liraglutide 3.0 mg (n = 142) or placebo (n = 140) as an adjunct to IBT.
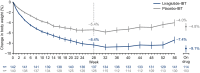
Results: At week 56, mean weight loss with liraglutide 3.0 mg plus IBT was 7.5% and 4.0% with placebo combined with IBT (estimated treatment difference [95% CI]-3.4% [-5.3% to -1.6%], P = 0.0003). Significantly more individuals on liraglutide 3.0 mg than placebo achieved ≥ 5% weight loss (61.5% vs. 38.8%; odds ratio [OR] 2.5% [1.5% to 4.1%], P = 0.0003), > 10% weight loss (30.5% vs. 19.8%; OR 1.8% [1.0% to 3.1%], P = 0.0469), and > 15% weight loss (18.1% vs. 8.9%; OR 2.3% [1.1% to 4.7%], P = 0.0311). Liraglutide 3.0 mg in combination with IBT was well tolerated, with no new safety signals identified.
Conclusions: In a primary care setting, Centers for Medicare and Medicaid Services-based IBT produced clinically meaningful weight loss at 56 weeks, enhanced by the addition of liraglutide 3.0 mg.
© 2020 The Authors. Obesity published by Wiley Periodicals, Inc. on behalf of The Obesity Society (TOS).
Conflict of interest statement
TW has received grants, on behalf of the University of Pennsylvania, from Novo Nordisk as well as honoraria from Novo Nordisk and WW (formerly Weight Watchers) for serving on scientific advisory boards. JST has received consulting fees/honoraria from Novo Nordisk. DS has received research grants from Novo Nordisk. DR has received grants from Obesinov SARL as well as consulting fees/honoraria from Novo Nordisk. MTL is an employee of Novo Nordisk and owns stock in the company. PA is an employee of Novo Nordisk. CJ is an employee of Novo Nordisk.
Figures



References
-
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(suppl 2):S102‐S138. - PMC - PubMed
-
- World Health Organization . Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Published February 16, 2018. Accessed August 8, 2019.
-
- National Institute for Health and Care Excellence . Obesity: identification, assessment and management. Clinical guideline [CG189]. https://www.nice.org.uk/guidance/cg189. Published November 2014. Accessed August 8, 2019.
-
- Webb VL, Wadden TA. Intensive lifestyle intervention for obesity: principles, practices, and results. Gastroenterology 2017;152:1752‐1764. - PubMed

