Iridium-Catalyzed Enantioselective Intermolecular Indole C2-Allylation
- PMID: 32091146
- PMCID: PMC7217203
- DOI: 10.1002/anie.202001956
Iridium-Catalyzed Enantioselective Intermolecular Indole C2-Allylation
Abstract
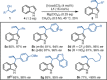
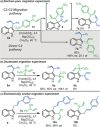
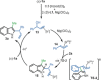
The enantioselective intermolecular C2-allylation of 3-substituted indoles is reported for the first time. This directing group-free approach relies on a chiral Ir-(P, olefin) complex and Mg(ClO4 )2 Lewis acid catalyst system to promote allylic substitution, providing the C2-allylated products in typically high yields (40-99 %) and enantioselectivities (83-99 % ee) with excellent regiocontrol. Experimental studies and DFT calculations suggest that the reaction proceeds via direct C2-allylation, rather than C3-allylation followed by in situ migration. Steric congestion at the indole-C3 position and improved π-π stacking interactions have been identified as major contributors to the C2-selectivity.
Keywords: DFT calculations; allylic substitution; enantioselective synthesis; indole; iridium.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- For a comprehensive overview, see: Katritzky A. R., Ramsden C. A., Scriven E. F. V., Taylor R. J. K., Comprehensive Heterocyclic Chemistry III, Elsevier, Oxford, 2008, and references therein (especially Volume 3, Ed.: C. A. Ramsden, G. Jones). For a review of the biomedical applications of indole derivatives, see:
- Kaushik N. K., Kaushik N., Attri P., Kumar N., Kim C. H., Verma A. K., Choi E. H., Molecules 2013, 18, 6620. - PMC - PubMed
-
- For a recent review, see: Zheng C., You S.-L., Nat. Prod. Rep. 2019, 36, 1589, and references therein. - PubMed
-
- For selected non-dearomative intermolecular asymmetric indole C3-allylic substitution reactions, see:
-
- Cheung H. Y., Yu W.-Y., Lam F. L., Au-Yeung T. T.-L., Zhou Z., Chan T. H., Chan A. S. C., Org. Lett. 2007, 9, 4295; - PubMed
-
- Liu W.-B., He H., Dai L.-X., You S.-L., Org. Lett. 2008, 10, 1815; - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

