Drug Development for Central Nervous System Diseases Using In vitro Blood-brain Barrier Models and Drug Repositioning
- PMID: 32091330
- PMCID: PMC7499354
- DOI: 10.2174/1381612826666200224112534
Drug Development for Central Nervous System Diseases Using In vitro Blood-brain Barrier Models and Drug Repositioning
Abstract
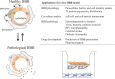
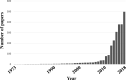
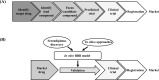
An important goal of biomedical research is to translate basic research findings into practical clinical implementation. Despite the advances in the technology used in drug discovery, the development of drugs for central nervous system diseases remains challenging. The failure rate for new drugs targeting important central nervous system diseases is high compared to most other areas of drug discovery. The main reason for the failure is the poor penetration efficacy across the blood-brain barrier. The blood-brain barrier represents the bottleneck in central nervous system drug development and is the most important factor limiting the future growth of neurotherapeutics. Meanwhile, drug repositioning has been becoming increasingly popular and it seems a promising field in central nervous system drug development. In vitro blood-brain barrier models with high predictability are expected for drug development and drug repositioning. In this review, the recent progress of in vitro BBB models and the drug repositioning for central nervous system diseases will be discussed.
Keywords: Blood-brain barrier; central nervous system disease; drug development; drug repositioning; neurotherapeutics; penetration efficacy..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures



Similar articles
-
From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery.Nat Rev Drug Discov. 2016 Apr;15(4):275-92. doi: 10.1038/nrd.2015.21. Epub 2016 Jan 22. Nat Rev Drug Discov. 2016. PMID: 26794270 Review.
-
[Research advances in brain-targeted nanoscale drug delivery system].Yao Xue Xue Bao. 2013 Oct;48(10):1532-43. Yao Xue Xue Bao. 2013. PMID: 24417079 Review. Chinese.
-
New experimental models of the blood-brain barrier for CNS drug discovery.Expert Opin Drug Discov. 2017 Jan;12(1):89-103. doi: 10.1080/17460441.2017.1253676. Epub 2016 Nov 7. Expert Opin Drug Discov. 2017. PMID: 27782770 Free PMC article. Review.
-
The blood-brain barrier: clinical implications for drug delivery to the brain.J R Coll Physicians Lond. 1994 Nov-Dec;28(6):502-6. J R Coll Physicians Lond. 1994. PMID: 7884704 Free PMC article. Review.
-
The blood-brain barrier: bottleneck in brain drug development.NeuroRx. 2005 Jan;2(1):3-14. doi: 10.1602/neurorx.2.1.3. NeuroRx. 2005. PMID: 15717053 Free PMC article. Review.
Cited by
-
Rapid and Widespread Distribution of Intranasal Small Extracellular Vesicles Derived from Mesenchymal Stem Cells throughout the Brain Potentially via the Perivascular Pathway.Pharmaceutics. 2023 Nov 3;15(11):2578. doi: 10.3390/pharmaceutics15112578. Pharmaceutics. 2023. PMID: 38004556 Free PMC article.
-
Recent Research Progress on the Chemical Constituents, Pharmacology, and Pharmacokinetics of Alpinae oxyphyllae Fructus.Molecules. 2024 Aug 18;29(16):3905. doi: 10.3390/molecules29163905. Molecules. 2024. PMID: 39202984 Free PMC article. Review.
-
Drug repurposing in amyotrophic lateral sclerosis (ALS).Expert Opin Drug Discov. 2025 Apr;20(4):447-464. doi: 10.1080/17460441.2025.2474661. Epub 2025 Mar 7. Expert Opin Drug Discov. 2025. PMID: 40029669 Free PMC article. Review.
-
The choroid plexus: a missing link in our understanding of brain development and function.Physiol Rev. 2023 Jan 1;103(1):919-956. doi: 10.1152/physrev.00060.2021. Epub 2022 Sep 29. Physiol Rev. 2023. PMID: 36173801 Free PMC article. Review.
-
Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity.Int J Mol Sci. 2022 Jan 27;23(3):1477. doi: 10.3390/ijms23031477. Int J Mol Sci. 2022. PMID: 35163400 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

