Randomized Trial of 2 Schedules of Meningococcal B Vaccine in Adolescents and Young Adults, Canada1
- PMID: 32091358
- PMCID: PMC7045834
- DOI: 10.3201/eid2603.190160
Randomized Trial of 2 Schedules of Meningococcal B Vaccine in Adolescents and Young Adults, Canada1
Abstract
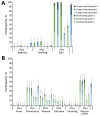
Emergency vaccination programs often are needed to control outbreaks of meningococcal disease caused by Neisseria meningitidis serogroup B (MenB) on college campuses. Such campaigns expend multiple campus and public health resources. We conducted a randomized, controlled, multicenter, observer-blinded trial comparing immunogenicity and tolerability of an accelerated vaccine schedule of 0 and 21 days to a longer interval of 0 and 60 days for 4-component MenB vaccine (MenB-4C) in students 17-25 years of age. At day 21 after the first MenB-4C dose, we observed protective human serum bactericidal titers >4 to MenB strains 5/99, H44/76, and NZ 98/254 in 98%-100% of participants. Geometric mean titers increased >22-fold over baseline. At day 180, >95% of participants sustained protective titers regardless of their vaccine schedule. The most common adverse event was injection site pain. An accelerated MenB-4C immunization schedule could be considered for rapid control of campus outbreaks.
Keywords: 4C-MenB; Canada; Neisseria meningitidis serogroup B; adolescents; bacteria; disease outbreaks; humans; meningitis/encephalitis; meningococcal vaccine; meningococcus serogroup B; prevention; student health services; vaccines; young adults.
Figures


References
-
- National Advisory Committee on Immunization. Canadian immunization guide for health professionals: meningococcal vaccine 2015. [cited 2018 November 28, 2018]. https://www.canada.ca/en/public-health/services/publications/healthy-liv...
-
- Schaffner W, Baker CJ, Bozof L, Engel J, Offit PA, Turner JC. Addressing the challenges of serogroup B meningococcal disease outbreaks on campuses: a report by the National Foundation for Infectious Diseases. Infect Dis Clin Pract. 2014;22:245–52. 10.1097/IPC.0000000000000197 - DOI
-
- US Centers for Disease Control and Prevention. Guidance for the evaluation and public health management of suspected outbreaks of meningococcal disease, version 1.0. Atlanta: The Centers; 2017.
-
- Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, et al.; Serogroup B Meningococcal Disease University Outbreak Group. Serogroup B Meningococcal Disease University Outbreak Group. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis. 2019;25:434–40. 10.3201/eid2503.181574 - DOI - PMC - PubMed
-
- Langley JM, MacDougall DM, Halperin BA, Swain A, Halperin SA, Top KA, et al. Rapid surveillance for health events following a mass meningococcal B vaccine program in a university setting: A Canadian Immunization Research Network study. Vaccine. 2016;34:4046–9. 10.1016/j.vaccine.2016.06.025 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

