Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
- PMID: 32091396
- PMCID: PMC7054001
- DOI: 10.7554/eLife.52690
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
Abstract
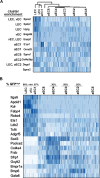
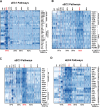
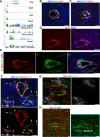
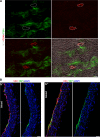
Despite the medical importance of G protein-coupled receptors (GPCRs), in vivo cellular heterogeneity of GPCR signaling and downstream transcriptional responses are not understood. We report the comprehensive characterization of transcriptomes (bulk and single-cell) and chromatin domains regulated by sphingosine 1-phosphate receptor-1 (S1PR1) in adult mouse aortic endothelial cells. First, S1PR1 regulates NFκB and nuclear glucocorticoid receptor pathways to suppress inflammation-related mRNAs. Second, S1PR1 signaling in the heterogenous endothelial cell (EC) subtypes occurs at spatially-distinct areas of the aorta. For example, a transcriptomically distinct arterial EC population at vascular branch points (aEC1) exhibits ligand-independent S1PR1/ß-arrestin coupling. In contrast, circulatory S1P-dependent S1PR1/ß-arrestin coupling was observed in non-branch point aEC2 cells that exhibit an inflammatory gene expression signature. Moreover, S1P/S1PR1 signaling regulates the expression of lymphangiogenic and inflammation-related transcripts in an adventitial lymphatic EC (LEC) population in a ligand-dependent manner. These insights add resolution to existing concepts of endothelial heterogeneity, GPCR signaling and S1P biology.
Keywords: Sphingosine 1-phosphate; arterial endothelium; chromatin; chromosomes; endothelial cells; gene expression; lymphatic endothelium; mouse; transcriptome.
Conflict of interest statement
EE, ML, LH, MV, AN, HN, AK, SS, MA, KH, RP, MK, WP, EC, CB No competing interests declared, TH received grant support from ONO Pharmaceuticals (2015-2018), has filed patent applications on ApoM, ApoM-Fc and HDL containing ApoM (US 62/545,629, PCT/US2018/000202, US 62/744,903, PCT/US2019/055831, US16/326,089, CA3034243, CN201780056922.6, JP 2019-530362, EPO 17851271.1), and has consulted for the following commercial entities: Astellas, Steptoe and Johnson, Gerson Lehrman Group Council, Janssen Research & Development, LLC, and Sun Pharma advanced research group (SPARC)
Figures












References
-
- Adey A, Morrison HG, Asan, Xun X, Kitzman JO, Turner EH, Stackhouse B, MacKenzie AP, Caruccio NC, Zhang X, Shendure J. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biology. 2010;11:R119. doi: 10.1186/gb-2010-11-12-r119. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

