Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial
- PMID: 32091594
- PMCID: PMC7566548
- DOI: 10.1093/jnci/djaa011
Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial
Abstract
Background: The authors investigated the durability of vaccine efficacy (VE) against human papillomavirus (HPV)16 or 18 infections and antibody response among nonrandomly assigned women who received a single dose of the bivalent HPV vaccine compared with women who received multiple doses and unvaccinated women.
Methods: HPV infections were compared between HPV16 or 18-vaccinated women aged 18 to 25 years who received one (N = 112), two (N = 62), or three (N = 1365) doses, and age- and geography-matched unvaccinated women (N = 1783) in the long-term follow-up of the Costa Rica HPV Vaccine Trial. Cervical HPV infections were measured at two study visits, approximately 9 and 11 years after initial HPV vaccination, using National Cancer Institute next-generation sequencing TypeSeq1 assay. VE and 95% confidence intervals (CIs) were estimated. HPV16 or 18 antibody levels were measured in all one- and two-dose women, and a subset of three-dose women, using a virus-like particle-based enzyme-linked immunosorbent assay (n = 448).
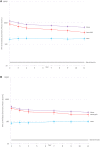
Results: Median follow-up for the HPV-vaccinated group was 11.3 years (interquartile range = 10.9-11.7 years) and did not vary by dose group. VE against prevalent HPV16 or 18 infection was 80.2% (95% CI = 70.7% to 87.0%) among three-dose, 83.8% (95% CI = 19.5% to 99.2%) among two-dose, and 82.1% (95% CI = 40.2% to 97.0%) among single-dose women. HPV16 or 18 antibody levels did not qualitatively decline between years four and 11 regardless of the number of doses given, although one-dose titers continue to be statistically significantly lower compared with two- and three-dose titers.
Conclusion: More than a decade after HPV vaccination, single-dose VE against HPV16 or 18 infection remained high and HPV16 or 18 antibodies remained stable. A single dose of bivalent HPV vaccine may induce sufficiently durable protection that obviates the need for more doses.
Published by Oxford University Press 2020. This work is written by US Government employees and is in the public domain in the US.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. - PubMed
-
- Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health. 2016;4(7):E453–E463. - PubMed
-
- Bruni L. Global vaccine uptake and projected cervical cancer disease reductions. HPV World. 2017;1(19):6–9.

