Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines
- PMID: 32092060
- PMCID: PMC7039414
- DOI: 10.1371/journal.pmed.1003038
Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines
Abstract
Background: HVTN 100 evaluated the safety and immunogenicity of an HIV subtype C pox-protein vaccine regimen, investigating a 12-month booster to extend vaccine-induced immune responses.
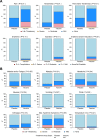
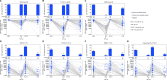
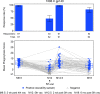
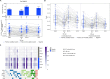
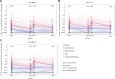
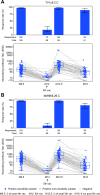
Methods and findings: A phase 1-2 randomized double-blind placebo-controlled trial enrolled 252 participants (210 vaccine/42 placebo; median age 23 years; 43% female) between 9 February 2015 and 26 May 2015. Vaccine recipients received ALVAC-HIV (vCP2438) alone at months 0 and 1 and with bivalent subtype C gp120/MF59 at months 3, 6, and 12. Antibody (IgG, IgG3 binding, and neutralizing) and CD4+ T-cell (expressing interferon-gamma, interleukin-2, and CD40 ligand) responses were evaluated at month 6.5 for all participants and at months 12, 12.5, and 18 for a randomly selected subset. The primary analysis compared IgG binding antibody (bAb) responses and CD4+ T-cell responses to 3 vaccine-matched antigens at peak (month 6.5 versus 12.5) and durability (month 12 versus 18) timepoints; IgG responses to CaseA2_gp70_V1V2.B, a primary correlate of risk in RV144, were also compared at these same timepoints. Secondary and exploratory analyses compared IgG3 bAb responses, IgG bAb breadth scores, neutralizing antibody (nAb) responses, antibody-dependent cellular phagocytosis, CD4+ polyfunctionality responses, and CD4+ memory sub-population responses at the same timepoints. Vaccines were generally safe and well tolerated. During the study, there were 2 deaths (both in the vaccine group and both unrelated to study products). Ten participants became HIV-infected during the trial, 7% (3/42) of placebo recipients and 3% (7/210) of vaccine recipients. All 8 serious adverse events were unrelated to study products. Less waning of immune responses was seen after the fifth vaccination than after the fourth, with higher antibody and cellular response rates at month 18 than at month 12: IgG bAb response rates to 1086.C V1V2, 21.0% versus 9.7% (difference = 11.3%, 95% CI = 0.6%-22.0%, P = 0.039), and ZM96.C V1V2, 21.0% versus 6.5% (difference = 14.5%, 95% CI = 4.1%-24.9%, P = 0.004). IgG bAb response rates to all 4 primary V1V2 antigens were higher 2 weeks after the fifth vaccination than 2 weeks after the fourth vaccination: 87.7% versus 75.4% (difference = 12.3%, 95% CI = 1.7%-22.9%, P = 0.022) for 1086.C V1V2, 86.0% versus 63.2% (difference = 22.8%, 95% CI = 9.1%-36.5%, P = 0.001) for TV1c8.2.C V1V2, 67.7% versus 44.6% (difference = 23.1%, 95% CI = 10.4%-35.7%, P < 0.001) for ZM96.C V1V2, and 81.5% versus 60.0% (difference = 21.5%, 95% CI = 7.6%-35.5%, P = 0.002) for CaseA2_gp70_V1V2.B. IgG bAb response rates to the 3 primary vaccine-matched gp120 antigens were all above 90% at both peak timepoints, with no significant differences seen, except a higher response rate to ZM96.C gp120 at month 18 versus month 12: 64.5% versus 1.6% (difference = 62.9%, 95% CI = 49.3%-76.5%, P < 0.001). CD4+ T-cell response rates were higher at month 18 than month 12 for all 3 primary vaccine-matched antigens: 47.3% versus 29.1% (difference = 18.2%, 95% CI = 2.9%-33.4%, P = 0.021) for 1086.C, 61.8% versus 38.2% (difference = 23.6%, 95% CI = 9.5%-37.8%, P = 0.001) for TV1.C, and 63.6% versus 41.8% (difference = 21.8%, 95% CI = 5.1%-38.5%, P = 0.007) for ZM96.C, with no significant differences seen at the peak timepoints. Limitations were that higher doses of gp120 were not evaluated, this study was not designed to investigate HIV prevention efficacy, and the clinical significance of the observed immunological effects is uncertain.
Conclusions: In this study, a 12-month booster of subtype C pox-protein vaccines restored immune responses, and slowed response decay compared to the 6-month vaccination.
Trial registration: ClinicalTrials.gov NCT02404311. South African National Clinical Trials Registry (SANCTR number: DOH--27-0215-4796).
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: CAD is a full-time employee at Sanofi Pasteur, shareholder Sanofi. SP is a full-time employee and shareholder at Sanofi Pasteur at the time of the study, now full-time employee at GSK group companies. MK is a full-time employee of GSK group companies, having GSK shares and stock options. OVDM is a full-time employee of GSK group companies, having GSK shares. MA, MNP, VLM are full-time employee of NIH/NIAID, which is the clinical trial sponsor. ZM is a a paid statistical advisor for PLOS Medicine.
Figures


References
-
- Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vcp1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis. 2012;12(7):531–7. 10.1016/S1473-3099(12)70088-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

