Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies
- PMID: 32092122
- PMCID: PMC7058349
- DOI: 10.1371/journal.ppat.1008083
Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies
Abstract
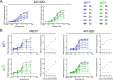
Antibody functions such as neutralization require recognition of antigen by the Fab region, while effector functions are additionally mediated by interactions of the Fc region with soluble factors and cellular receptors. The efficacy of individual antibodies varies based on Fab domain characteristics, such as affinity for antigen and epitope-specificity, and on Fc domain characteristics that include isotype, subclass, and glycosylation profile. Here, a series of HIV-specific antibody subclass and hinge variants were constructed and tested to define those properties associated with differential effector function. In the context of the broadly neutralizing CD4 binding site-specific antibody VRC01 and the variable loop (V3) binding antibody 447-52D, hinge truncation and extension had a considerable impact on the magnitude of phagocytic activity of both IgG1 and IgG3 subclasses. The improvement in phagocytic potency of antibodies with extended hinges could not be attributed to changes in either intrinsic antigen or antibody receptor affinity. This effect was specific to phagocytosis and was generalizable to different phagocytes, at different effector cell to target ratios, for target particles of different size and composition, and occurred across a range of antibody concentrations. Antibody dependent cellular cytotoxicity and neutralization were generally independent of hinge length, and complement deposition displayed variable local optima. In vivo stability testing showed that IgG molecules with altered hinges can exhibit similar biodistribution and pharmacokinetic profiles as IgG1. Overall, these results suggest that when high phagocytic activity is desirable, therapeutic antibodies may benefit from being formatted as human IgG3 or engineered IgG1 forms with elongated hinges.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

