Homozygous STAT2 gain-of-function mutation by loss of USP18 activity in a patient with type I interferonopathy
- PMID: 32092142
- PMCID: PMC7201920
- DOI: 10.1084/jem.20192319
Homozygous STAT2 gain-of-function mutation by loss of USP18 activity in a patient with type I interferonopathy
Abstract
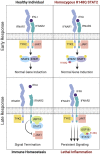
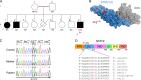
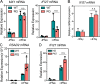
Type I interferonopathies are monogenic disorders characterized by enhanced type I interferon (IFN-I) cytokine activity. Inherited USP18 and ISG15 deficiencies underlie type I interferonopathies by preventing the regulation of late responses to IFN-I. Specifically, USP18, being stabilized by ISG15, sterically hinders JAK1 from binding to the IFNAR2 subunit of the IFN-I receptor. We report an infant who died of autoinflammation due to a homozygous missense mutation (R148Q) in STAT2. The variant is a gain of function (GOF) for induction of the late, but not early, response to IFN-I. Surprisingly, the mutation does not enhance the intrinsic activity of the STAT2-containing transcriptional complex responsible for IFN-I-stimulated gene induction. Rather, the STAT2 R148Q variant is a GOF because it fails to appropriately traffic USP18 to IFNAR2, thereby preventing USP18 from negatively regulating responses to IFN-I. Homozygosity for STAT2 R148Q represents a novel molecular and clinical phenocopy of inherited USP18 deficiency, which, together with inherited ISG15 deficiency, defines a group of type I interferonopathies characterized by an impaired regulation of late cellular responses to IFN-I.
© 2020 Gruber et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Cavaco B.M., Canaff L., Nolin-Lapalme A., Vieira M., Silva T.N., Saramago A., Domingues R., Rutter M.M., Hudon J., Gleason J.L., et al. . 2018. Homozygous Calcium-Sensing Receptor Polymorphism R544Q Presents as Hypocalcemic Hypoparathyroidism. J. Clin. Endocrinol. Metab. 103:2879–2888. 10.1210/jc.2017-02407 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

