Influenza virus NS1- C/EBPβ gene regulatory complex inhibits RIG-I transcription
- PMID: 32092305
- PMCID: PMC10773002
- DOI: 10.1016/j.antiviral.2020.104747
Influenza virus NS1- C/EBPβ gene regulatory complex inhibits RIG-I transcription
Abstract
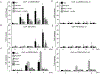
Influenza virus non-structural protein 1 (NS1) counteracts host antiviral innate immune responses by inhibiting Retinoic acid inducible gene-I (RIG-I) activation. However, whether NS1 also specifically regulates RIG-I transcription is unknown. Here, we identify a CCAAT/Enhancer Binding Protein beta (C/EBPβ) binding site in the RIG-I promoter as a repressor element, and show that NS1 promotes C/EBPβ phosphorylation and its recruitment to the RIG-I promoter as a C/EBPβ/NS1 complex. C/EBPβ overexpression and siRNA knockdown in human lung epithelial cells resulted in suppression and activation of RIG-I expression respectively, implying a negative regulatory role of C/EBPβ. Further, C/EBPβ phosphorylation, its interaction with NS1 and occupancy at the RIG-I promoter was associated with RIG-I transcriptional inhibition. These findings provide an important insight into the molecular mechanism by which influenza NS1 commandeers RIG-I transcriptional regulation and suppresses host antiviral responses.
Keywords: C/EBPβ; Influenza; NS1; RIG-I; Transcriptional regulation.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflicts of interest with the contents of this article. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of Centers for Disease Control and Prevention.
Figures


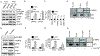

References
-
- Anastasina M, Le May N, Bugai A, Fu Y, Soderholm S, Gaelings L, Ohman T, Tynell J, Kyttanen S, Barboric M, Nyman TA, Matikainen S, Julkunen I, Butcher SJ, Egly JM, Kainov DE, 2016. Influenza virus NS1 protein binds cellular DNA to block transcription of antiviral genes. Biochimica et biophysica acta 1859, 1440–1448. - PubMed
-
- Baeuerle PA, 1991. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochimica et biophysica acta 1072, 63–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

