New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story
- PMID: 32092897
- PMCID: PMC7157223
- DOI: 10.3390/tropicalmed5010029
New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story
Abstract
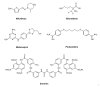
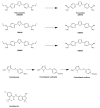
The twentieth century ended with human African trypanosomiasis (HAT) epidemics raging across many parts of Africa. Resistance to existing drugs was emerging, and many programs aiming to contain the disease had ground to a halt, given previous success against HAT and the competing priorities associated with other medical crises ravaging the continent. A series of dedicated interventions and the introduction of innovative routes to develop drugs, involving Product Development Partnerships, has led to a dramatic turnaround in the fight against HAT caused by Trypanosoma brucei gambiense. The World Health Organization have been able to optimize the use of existing tools to monitor and intervene in the disease. A promising new oral medication for stage 1 HAT, pafuramidine maleate, ultimately failed due to unforeseen toxicity issues. However, the clinical trials for this compound demonstrated the possibility of conducting such trials in the resource-poor settings of rural Africa. The Drugs for Neglected Disease initiative (DNDi), founded in 2003, has developed the first all oral therapy for both stage 1 and stage 2 HAT in fexinidazole. DNDi has also brought forward another oral therapy, acoziborole, potentially capable of curing both stage 1 and stage 2 disease in a single dosing. In this review article, we describe the remarkable successes in combating HAT through the twenty first century, bringing the prospect of the elimination of this disease into sight.
Keywords: acoziborole; chemotherapy; elimination; fexinidazole; human African trypanosomiasis; pafuramidine; sleeping sickness.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- [(accessed on 12 January 2020)]; Available online: https://www.who.int/trypanosomiasis_african/en/
-
- Priotto G., Kasparian S., Mutombo W., Ngouama D., Ghorashian S., Arnold U., Ghabri S., Baudin E., Buard V., Kazadi-Kyanza S., et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicenter, randomised, phase III, non-inferiority trial. Lancet. 2009;374:56–64. doi: 10.1016/S0140-6736(09)61117-X. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

