3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo
- PMID: 32092907
- PMCID: PMC7074400
- DOI: 10.3390/md18020123
3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo
Abstract
Structure-based tissue engineering requires large-scale 3D cell/tissue manufacture technologies, to produce biologically active scaffolds. Special attention is currently paid to naturally pre-designed scaffolds found in skeletons of marine sponges, which represent a renewable resource of biomaterials. Here, an innovative approach to the production of mineralized scaffolds of natural origin is proposed. For the first time, a method to obtain calcium carbonate deposition ex vivo, using living mollusks hemolymph and a marine-sponge-derived template, is specifically described. For this purpose, the marine sponge Aplysin aarcheri and the terrestrial snail Cornu aspersum were selected as appropriate 3D chitinous scaffold and as hemolymph donor, respectively. The formation of calcium-based phase on the surface of chitinous matrix after its immersion into hemolymph was confirmed by Alizarin Red staining. A direct role of mollusks hemocytes is proposed in the creation of fine-tuned microenvironment necessary for calcification ex vivo. The X-ray diffraction pattern of the sample showed a high CaCO3 amorphous content. Raman spectroscopy evidenced also a crystalline component, with spectra corresponding to biogenic calcite. This study resulted in the development of a new biomimetic product based on ex vivo synthetized ACC and calcite tightly bound to the surface of 3D sponge chitin structure.
Keywords: biomineralization; calcite; chitin; hemocytes; hemolymph; scaffold; sponges.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



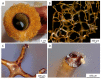



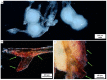






References
-
- Morganti P., Coltelli M.B., Santi S. Biobased tissues for innovative cosmetic products: Polybioskin as an EU research project. Glob. J. Nanomedicine. 2018;3:1–6.
-
- Klinger C., Żółtowska-Aksamitowska S., Wysokowski M., Tsurkan M.V., Galli R., Petrenko I., Machałowski T., Ereskovsky A., Martinović R., Muzychka L., et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs. 2019;17:131. doi: 10.3390/md17020131. - DOI - PMC - PubMed
-
- Zhang X., Vecchio K.S. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front. Mater. Sci. 2013;7:103–117. doi: 10.1007/s11706-013-0204-x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

