Targeting Mitochondrial Calcium Uptake with the Natural Flavonol Kaempferol, to Promote Metabolism/Secretion Coupling in Pancreatic β-cells
- PMID: 32093050
- PMCID: PMC7071504
- DOI: 10.3390/nu12020538
Targeting Mitochondrial Calcium Uptake with the Natural Flavonol Kaempferol, to Promote Metabolism/Secretion Coupling in Pancreatic β-cells
Abstract
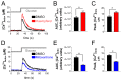
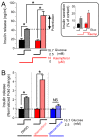
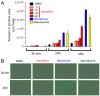
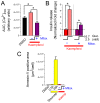
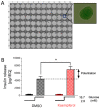
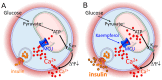
Pancreatic β-cells secrete insulin to lower blood glucose, following a meal. Maintenance of β-cell function is essential to preventing type 2 diabetes. In pancreatic β-cells, mitochondrial matrix calcium is an activating signal for insulin secretion. Recently, the molecular identity of the mitochondrial calcium uniporter (MCU), the transporter that mediates mitochondrial calcium uptake, was revealed. Its role in pancreatic β-cell signal transduction modulation was clarified, opening new perspectives for intervention. Here, we investigated the effects of a mitochondrial Ca2+-targeted nutritional intervention strategy on metabolism/secretion coupling, in a model of pancreatic insulin-secreting cells (INS-1E). Acute treatment of INS-1E cells with the natural plant flavonoid and MCU activator kaempferol, at a low micromolar range, increased mitochondrial calcium rise during glucose stimulation, without affecting the expression level of the MCU and with no cytotoxicity. Enhanced mitochondrial calcium rises potentiated glucose-induced insulin secretion. Conversely, the MCU inhibitor mitoxantrone inhibited mitochondrial Ca2+ uptake and prevented both glucose-induced insulin secretion and kaempferol-potentiated effects. The kaempferol-dependent potentiation of insulin secretion was finally validated in a model of a standardized pancreatic human islet. We conclude that the plant product kaempferol activates metabolism/secretion coupling in insulin-secreting cells by modulating mitochondrial calcium uptake.
Keywords: calcium; insulin; kaempferol; mitochondria; mitoxantrone; polyphenols; β-cell.
Conflict of interest statement
The authors are employees of Nestlé Research, which is part of the Société des Produits Nestlé SA.
Figures

References
-
- Alam M.R., Groschner L.N., Parichatikanond W., Kuo L., Bondarenko A.I., Rost R., Waldeck-Weiermair M., Malli R., Graier W.F. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. J. Biol. Chem. 2012;287:34445–34454. doi: 10.1074/jbc.M112.392084. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

