Computational and Experimental 1H-NMR Study of Hydrated Mg-Based Minerals
- PMID: 32093106
- PMCID: PMC7070456
- DOI: 10.3390/molecules25040933
Computational and Experimental 1H-NMR Study of Hydrated Mg-Based Minerals
Abstract
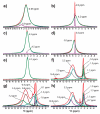
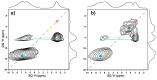
Magnesium oxide (MgO) can convert to different magnesium-containing compounds depending on exposure and environmental conditions. Many MgO-based phases contain hydrated species allowing 1H-nuclear magnetic resonance (NMR) spectroscopy to be used in the characterization and quantification of proton-containing phases; however, surprisingly limited examples have been reported. Here, 1H-magic angle spinning (MAS) NMR spectra of select Mg-based minerals are presented and assigned. These experimental results are combined with computational NMR density functional theory (DFT) periodic calculations to calibrate the predicted chemical shielding results. This correlation is then used to predict the NMR shielding for a series of different MgO hydroxide, magnesium chloride hydrate, magnesium perchlorate, and magnesium cement compounds to aid in the future assignment of 1H-NMR spectra for complex Mg phases.
Keywords: 1H-NMR; DFT; GIPAW; chemical shift; hydroxylated; magnesium minerals; magnesium oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Amaral L., Oliveira I., Salomao R., Frollini E., Pandolfelli V. Temperature and Common-Ion Effect on Magnesium Oxide (MgO) Hydration. Ceram. Int. 2010;36:1047–1054. doi: 10.1016/j.ceramint.2009.12.009. - DOI
-
- Chizallet C., Costentin G., Lauron-Pernot H., Che M., Bonhomme C., Maquet J., Delbecq F., Sautet P. Study of the Structure of OH Groups on MgO by 1D and 2D 1H MAS NMR Combined with DFT Cluster Calculations. J. Phys. Chem. C. 2007;111:18279–18287. doi: 10.1021/jp077089g. - DOI
-
- Chizallet C., Costentin G., Lauron-Pernot H., Maquet J., Che M. 1H MAS NMR Study of the Coordination of Hydroxyl Groups Generated Upon Adsorption of H2O and CD3OH on Clean MgO Surfaces. Appl. Catal. A. 2006;307:239–244. doi: 10.1016/j.apcata.2006.03.056. - DOI
-
- Liu Z., De Schutter G., Deng D., Yu Z. Micro-Analysis of the Role of Interfacial Transition Zone in “Salt Weathering” on Concrete. Constr. Build. Mater. 2010;24:2052–2059. doi: 10.1016/j.conbuildmat.2010.04.053. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

