Stiffer Matrix Accelerates Migration of Hepatocellular Carcinoma Cells through Enhanced Aerobic Glycolysis Via the MAPK-YAP Signaling
- PMID: 32093118
- PMCID: PMC7072284
- DOI: 10.3390/cancers12020490
Stiffer Matrix Accelerates Migration of Hepatocellular Carcinoma Cells through Enhanced Aerobic Glycolysis Via the MAPK-YAP Signaling
Abstract
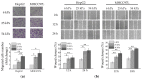
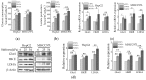
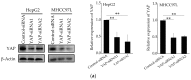
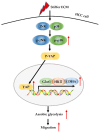
Increased extracellular matrix (ECM) stiffness and metabolic reprogramming of cancer cells are two fundamental mediators of tumor progression, including hepatocellular carcinoma (HCC). Yet, the correlation between ECM stiffness and excessive aerobic glycolysis in promoting the development of HCC remains unknown. Here, we demonstrated that stiffer ECM promotes HCC cell migration depending on their accelerated aerobic glycolysis. Our results also indicated that stiffer ECM-induced YAP activation plays a major role in promoting aerobic glycolysis of HCC cells. Moreover, we showed that JNK and p38 MAPK signaling are critical for mediating YAP activation in HCC cells. Together, our findings established that the MAPK-YAP signaling cascade that act as a mechanotransduction pathway is essential for promoting HCC cell aerobic glycolysis and migration in response to ECM stiffness.
Keywords: ECM stiffness; aerobic glycolysis; hepatocellular carcinoma; mechanotransduction; migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

